Coronary artery disease (CAD) is found in 30%-50% of patients with severe aortic stenosis (AS) undergoing treatment. The best management of CAD in AS patients undergoing transcatheter aortic valve implantation (TAVI) is still unclear. We investigated the clinical impact of the extent of jeopardized myocardium in patients with concomitant CAD and severe AS treated by TAVI.
MethodsConsecutive patients who underwent TAVI procedures at our hospital were identified. In the presence of CAD, the myocardium jeopardized before TAVI was graded using the British Cardiovascular Intervention Society (BCIS) jeopardy score (JS). The study population was divided in 3 groups: patients without concomitant CAD (no-CAD), patients with CAD and BCIS-JS ≤ 4 (CAD BCIS-JS ≤ 4) and patients with concomitant CAD and BCIS-JS> 4 (CAD BCIS-JS> 4). The primary study endpoint was major adverse cardiovascular and cerebrovascular events (MACCE).
ResultsA total of 403 patients entered the study: 223 no-CAD, 94 CAD BCIS-JS ≤ 4 and 86 CAD BCIS-JS> 4. At> 3 months of follow-up [range 104–3296 days], patients without CAD and CAD patients with BCIS-JS ≤ 4 had better survival free from MACCE compared with those with less extensive revascularization (BCIS-JS> 4) (P=.049). This result was driven by a significant reduction in death (P=.031). On multivariate analysis, residual BCIS-JS ≤ 4 and NYHA class III-IV independently predicted MACCE.
ConclusionsIn patients with concomitant CAD and severe AS, the extent of jeopardized myocardium before TAVI impacts on clinical outcomes.
Keywords
Coronary artery disease (CAD) is a common finding in patients with degenerative aortic valve stenosis (AS).1 Thus, CAD has been reported in as high as> 50% of AS patients undergoing both surgical treatment2 or transcatheter aortic valve intervention (TAVI).3–5 Usually, when CAD is found in AS patients scheduled for surgery, it is treated by coronary artery bypass grafting (CABG) performed at the time of surgical aortic valve replacement.6 Despite the high prevalence of CAD in patients treated with TAVI, the management strategy of concomitant CAD in these patients remains an area of considerable uncertainty. Severe CAD is associated with increased mortality after TAVI,7,8 but percutaneous coronary interventions (PCI) in TAVI patients might be challenging. Current guidelines recommend the performance of PCI before or at the time of TAVI in patients with a coronary artery diameter stenosis> 70% in proximal segments based on a few observational studies.9 In this retrospective observational study, we investigated the clinical impact of the extent of jeopardized myocardium in patients with concomitant CAD and severe AS treated by TAVI.
METHODSStudy population selectionWe conducted a retrospective observational study including consecutive patients who underwent TAVI at our center in a 9-year period (from October 2010 to December 2019).
According to the standard practice of our center, all patients were referred for TAVI on the basis of formal, multidisciplinary, Heart Team discussion. Clinical data and procedure details were prospectively entered into the TAVI-dedicated section of an electronic database that allowed previous assessment of the impact of EuroSCORE on coronary interventions10 and the safety of transradial procedures.11
Patients’ surgical risk was graded according to the logistic EuroSCORE and the Society of Thoracic Surgeons (STS) predicted operative mortality at the time of Heart Team consultation. TAVI risk was graded according to the STS/American College of Cardiology Transcatheter Valve Therapy using the on-line TAVI in-hospital mortality risk calculator.12
All patients signed a dedicated informed consent form to undergo the scheduled procedure which includes authorization for database insertion and clinical follow-up assessment.
For the present study, all patients who underwent a TAVI procedure in our institution between October 2010 and December 2019 were retrospectively screened.
Coronary artery disease assessmentAll patients underwent coronary evaluation before TAVI. The vast majority of the patients received coronary angiography (not performed only in the case of nonobstructive coronary arteries on computed tomography coronary angiography, 80 patients). Patients were classified as having CAD if they fulfilled 1 of the following conditions: coronary stenosis> 70% in at least 1 major epicardial coronary artery or prior coronary revascularization by either PCI or CABG. For each patient with concomitant CAD, the jeopardized myocardium before the TAVI was graded using the British Cardiovascular Intervention Society (BCIS) Jeopardy Score (JS).13 The extent of coronary revascularization and the use of fractional flow reserve to guide the revascularization were left at the operator's discretion.
Clinical follow-up and primary endpointFor all enrolled patients, clinical records were carefully evaluated, and clinical follow-up was obtained (as in-person visits, telephone interviews, and medical notes from any hospital admission or outpatient visits). Nonfatal myocardial infarction (MI) during follow-up was defined as the rise and fall of cardiac enzymes (usually serum high-sensitivity troponin I) in the presence of electrocardiogram signs or symptoms compatible with myocardial ischemia, as described in the fourth universal definition of MI14 as enrollment started before the publication of the fourth universal definition. Stroke was defined as any new, permanent, global, or focal neurological deficit ascertained by a standard neurological examination, lasting longer than 24hours or less if evidence of cerebral infarction was obtained by imaging. Coronary revascularization was defined as any coronary revascularization (either PCI or CABG) performed after TAVI regardless of the fact that it was related to a previously treated or untreated segment.
The primary study endpoint was major adverse cardiovascular and cerebrovascular events (MACCE) defined as the composite of death, nonfatal MI, nonfatal stroke, and coronary revascularization. The individual components of the primary endpoint constituted the secondary endpoints.
Statistical analysisContinuous variables are presented as mean± standard deviation and categorical variables as numbers and percentages. Comparisons of continuous variables across different groups were performed using the Student t test or ANOVA test (as appropriate). Categorical variables were evaluated using the chi-square test or Fisher exact test, as appropriate. A Cox regression analysis was performed to identify the independent predictors of the primary endpoint among the main baseline characteristics (age, sex, cardiovascular risk factors, renal failure, prior MI, prior PCI, prior cardiac surgery, mean aortic gradient, aortic valve area, ejection fraction, NYHA class III-IV, STS mortality, TAVI score, coronary anatomy, and BCIS-JS). Adjusted hazard ratios (HR) with associated 95% confidence interval (95%CI) were calculated for the significant primary endpoint predictors and corresponding adjusted survival curves were determined. The subdistributions of a competing risk with the Fine-Gray test were calculated for the competing risk events included in the secondary endpoints.15 A 2-tailed, P-value <.05 was established as the level of statistical significance. All statistical analyses were performed using SPSS software v22.0 (IBM Corporation, United States) and SAS software 9 (SAS Institute, United States).
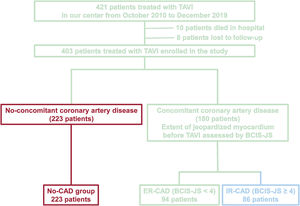
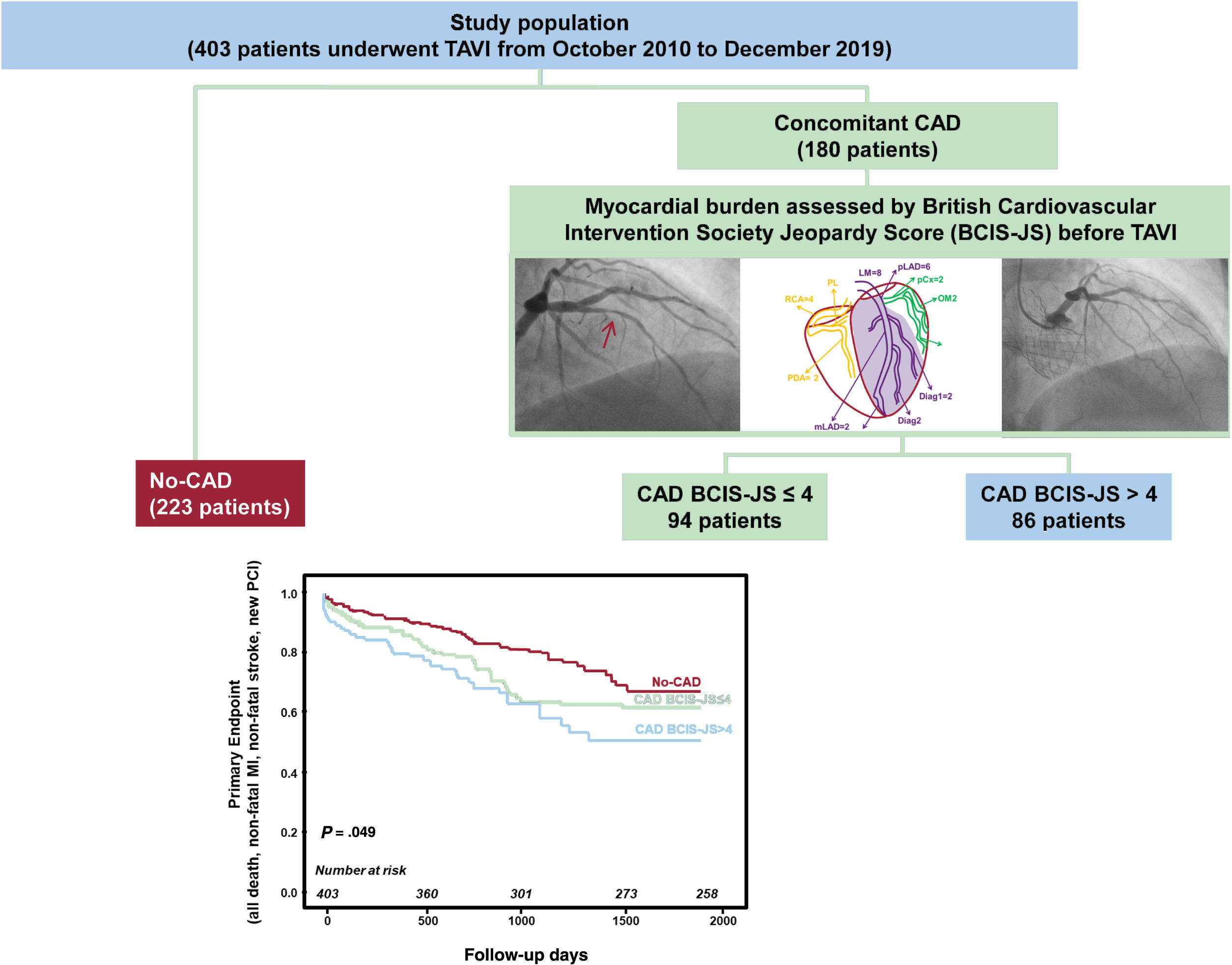
RESULTSPatient populationOut of 421 patients who underwent TAVI in our hospital from October 2010 to December 2019, 18 patients were excluded (10 died in hospital and 8 patients were lost to follow-up). Thus, 403 patients (180 with concomitant CAD) were enrolled in the study. The median BCIS-JS before TAVI was 4.0 [IQR=0.7]. Thus, the study population was divided in 3 groups: patients without concomitant CAD (no-CAD), patients with CAD and BCIS-JS before TAVI ≤ 4 (CAD BCIS-JS ≤ 4), and patients with concomitant CAD and BCIS-JS before TAVI> 4 (CAD BCIS-JS> 4). By combining the presence or absence of CAD and BCI-JS before TAVI (≤ or> 4), the study population was divided in the following 3 groups: 223 patients with no-CAD, 94 patients with ER-CAD, and 86 patients with IR-CAD (figure 1).
Study flow chart. CAD, coronary artery disease; BCIS-JS, British Cardiovascular Intervention Society Jeopardy Score; ER-CD, extensively revascularized coronary artery disease; IR-CAD, incompletely revascularized coronary artery disease; TAVI, transcatheter aortic valve implantation.
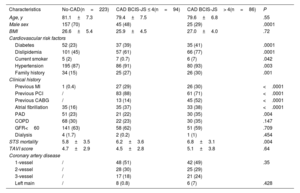
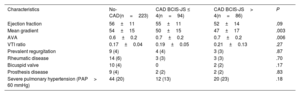
The baseline characteristics of the patients are listed in table 1, showing marked differences between the 3 study subgroups. Among these, in CAD BCIS-JS> 4, male sex was less prevalent (29% vs 48% vs 70%, P <.0001) and the incidence of peripheral artery disease was higher (35% vs 22% vs 23%, P=.004) compared with CAD BCIS-JS ≤ 4 and no-CAD. As expected, the incidence of all cardiovascular risk factors was more frequent in the CAD BCIS-JS> 4 and CAD BCIS-JS ≤ 4 groups compared with no-CAD group (diabetes 41% vs 39% vs 23%, P <.0001; dyslipidemia 77% vs 61% vs 45%, P <.0001; current smoker 7% vs 0.7% vs 2%, P=.042; hypertension 93% vs 91% vs 87%, P=.003; family history 30% vs 27% vs 15%, P=.001). The incidence of atrial fibrillation was higher in the CAD BCIS-JS> 4 and CAD BCIS-JS ≤ 4 groups compared with the no-CAD group (38% vs 37% vs 16%, P <.0001). Similarly, the STS mortality score was higher in patients with concomitant CAD (CAD BCIS-JS> 4 and CAD BCIS-JS ≤ 4) compared with no-CAD patients (6.8±3.1 vs 6.2±3.6 vs 5.8±3.5, P=.004). In contrast, TAVI score did not differ between the 3 study subgroups. Of note, among patients with concomitant CAD, there were no differences in the distribution of coronary disease. Echocardiography characteristics are reported in table 2. In no-CAD patients, the mean aortic gradient was higher (54±15 vs 50±15 vs 47±17, P=.003) and the aortic valve area was smaller (0.6±0.2 vs 0.7±0.2 vs 0.7±0.2, P=.006) compared with patients with concomitant CAD (both CAD BCIS-JS> 4 and CAD BCIS-JS ≤ 4).
Baseline characteristics of the study population
| Characteristics | No-CAD(n=223) | CAD BCIS-JS ≤ 4(n=94) | CAD BCIS-JS> 4(n=86) | P |
|---|---|---|---|---|
| Age, y | 81.1±7.3 | 79.4±7.5 | 79.6±6.8 | .55 |
| Male sex | 157 (70) | 45 (48) | 25 (29) | .0001 |
| BMI | 26.6±5.4 | 25.9±4.5 | 27.0±4.0 | .72 |
| Cardiovascular risk factors | ||||
| Diabetes | 52 (23) | 37 (39) | 35 (41) | .0001 |
| Dislipidemia | 101 (45) | 57 (61) | 66 (77) | .0001 |
| Current smoker | 5 (2) | 7 (0.7) | 6 (7) | .042 |
| Hypertension | 195 (87) | 86 (91) | 80 (93) | .003 |
| Family history | 34 (15) | 25 (27) | 26 (30) | .001 |
| Clinical history | ||||
| Previous MI | 1 (0.4) | 27 (29) | 26 (30) | <.0001 |
| Previous PCI | / | 83 (88) | 61 (71) | <.0001 |
| Previous CABG | / | 13 (14) | 45 (52) | <.0001 |
| Atrial fibrillation | 35 (16) | 35 (37) | 33 (38) | <.0001 |
| PAD | 51 (23) | 21 (22) | 30 (35) | .004 |
| COPD | 68 (30) | 22 (23) | 30 (35) | .147 |
| GFR<60 | 141 (63) | 58 (62) | 51 (59) | .709 |
| Dialysis | 4 (1.7) | 2 (0.2) | 1 (1) | .454 |
| STS mortality | 5.8±3.5 | 6.2±3.6 | 6.8±3.1 | .004 |
| TAVI score | 4.7±2.9 | 4.5±2.8 | 5.1±3.8 | .64 |
| Coronary artery disease | ||||
| 1-vessel | / | 48 (51) | 42 (49) | .35 |
| 2-vessel | / | 28 (30) | 25 (29) | |
| 3-vessel | / | 17 (18) | 21 (24) | |
| Left main | / | 8 (0.8) | 6 (7) | .428 |
BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Data are expressed as No. (%) or mean±standard deviation.
The coronary artery disease population was stratified according to myocardial burden, assessed by the British Cardiovascular Intervention Society Jeopardy Score (BCIS-JS), before transcatheter aortic valve implantation.
Echocardiographic characteristics of study population
| Characteristics | No-CAD(n=223) | CAD BCIS-JS ≤ 4(n=94) | CAD BCIS-JS> 4(n=86) | P |
|---|---|---|---|---|
| Ejection fraction | 56±11 | 55±11 | 52±14 | .09 |
| Mean gradient | 54±15 | 50±15 | 47±17 | .003 |
| AVA | 0.6±0.2 | 0.7±0.2 | 0.7±0.2 | .006 |
| VTI ratio | 0.17±0.04 | 0.19±0.05 | 0.21±0.13 | .27 |
| Prevalent regurgitation | 9 (4) | 4 (4) | 3 (3) | .87 |
| Rheumatic disease | 14 (6) | 3 (3) | 3 (3) | .70 |
| Bicuspid valve | 10 (4) | 0 | 2 (2) | .17 |
| Prosthesis disease | 9 (4) | 2 (2) | 2 (2) | .83 |
| Severe pulmonary hypertension (PAP> 60 mmHg) | 44 (20) | 12 (13) | 20 (23) | .18 |
PAPS, pulmonary arterial pressure.
Data are expressed as No. (%) or mean±standard deviation.
The coronary artery disease population was stratified according to myocardial burden, assessed by British Cardiovascular Intervention Society Jeopardy Score (BCIS-JS), before TAVI.
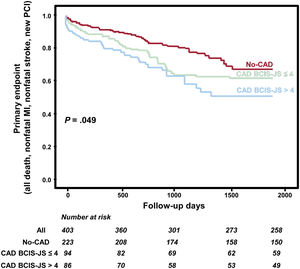
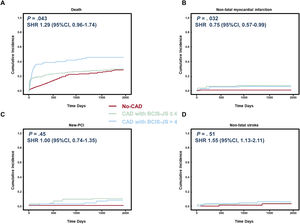
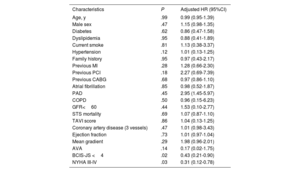
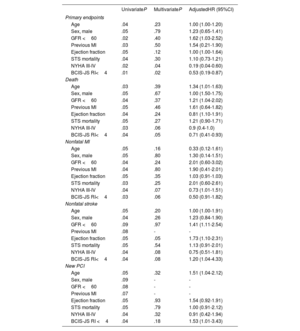
All patients had a follow-up of> 3 months [range 104-3 296 days] and the mean length of follow-up was ∼5 years. figure 2 reports the survival curves (table 3) in the 3 study groups showing that CAD BCIS-JS ≤ 4 had event-rates comparable to the no-CAD group and significantly better compared with CAD BCIS-JS> 4 patients (P=.049). As shown in figure 3, this result was driven by a statistically significantly higher occurrence of death in patients with CAD BCIS-JS> 4 compared with those with CAD BCIS-JS ≤ 4 and no-CAD (SHR, 1.29; 95%CI, 0.96-1.74; P=.043). Of note, the incidence of nonfatal MI was higher in patients with concomitant CAD independently of the extent of jeopardized myocardium achieved before TAVI compared with no-CAD patients (SHR, 1.74; 95%CI,1.28-2.50; P=.032). The Cox regression analysis identified BJS <4 (HR, 0.43; 95%CI, 0.21-0.90; P=.02) and NYHA III-IV class (HR, 0.31; 95%CI, 0.12-0.78; P=.03) as independent predictors of the primary endpoint among the main baseline characteristics (table 3). On multivariate analysis (table 4) including all the main baseline characteristics, BCIS-JS ≤ 4 (HR, 0.53; 95%CI, 0.19-0.87; P=.02) and NYHA class III-IV (HR, 0.19; 95%CI, 0.04-0.60; P=.04) were independent predictors of the primary endpoint.
Primary endpoint according to extent of jeopardized myocardium before transcatheter aortic valve implantation. The figure shows the event-free survival curves in the study population. The coronary artery disease population was stratified according to myocardial burden, assessed by the British Cardiovascular Intervention Society Jeopardy Score (BCIS-JS) before TAVI. BCIS-JS, British Cardiovascular Intervention Society Jeopardy Score; CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary interventions; TAVI, transcatheter aortic valve implantation.
Cox regression analysis for independent predictors of primary endpoint
| Characteristics | P | Adjusted HR (95%CI) |
|---|---|---|
| Age, y | .99 | 0.99 (0.95-1.39) |
| Male sex | .47 | 1.15 (0.98-1.35) |
| Diabetes | .62 | 0.86 (0.47-1.58) |
| Dyslipidemia | .95 | 0.88 (0.41-1.89) |
| Current smoke | .81 | 1.13 (0.38-3.37) |
| Hypertension | .12 | 1.01 (0.13-1.25) |
| Family history | .95 | 0.97 (0.43-2.17) |
| Previous MI | .28 | 1.28 (0.66-2.30) |
| Previous PCI | .18 | 2.27 (0.69-7.39) |
| Previous CABG | .68 | 0.97 (0.86-1.10) |
| Atrial fibrillation | .85 | 0.98 (0.52-1.87) |
| PAD | .45 | 2.95 (1.45-5.97) |
| COPD | .50 | 0.96 (0.15-6.23) |
| GFR<60 | .44 | 1.53 (0.10-2.77) |
| STS mortality | .69 | 1.07 (0.87-1.10) |
| TAVI score | .86 | 1.04 (0.13-1.25) |
| Coronary artery disease (3 vessels) | .47 | 1.01 (0.98-3.43) |
| Ejection fraction | .73 | 1.01 (0.97-1.04) |
| Mean gradient | .29 | 1.98 (0.96-2.01) |
| AVA | .14 | 0.17 (0.02-1.75) |
| BCIS-JS <4 | .02 | 0.43 (0.21-0.90) |
| NYHA III-IV | .03 | 0.31 (0.12-0.78) |
AVA, aortic valve area; BCIS-JS, British Cardiovascular Intervention Society-Jeopardy Score; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; MI, myocardial infarction; NYHA, Yew York Heart Association; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Single component of the primary endpoint according to extent of jeopardized myocardium before transcatheter aortic valve implantation. The figure shows the cumulative incidence function curve using Fine-Grey competing risk model of the individual components of the primary endpoint in the study population. A, death; B, nonfatal myocardial infarction; C, new-percutaneous coronary interventions; D, nonfatal stroke). The coronary artery disease population was stratified according to myocardial burden, assessed by the British Cardiovascular Intervention Society Jeopardy Score (BCIS-JS) before TAVI. CAD, coronary artery disease; PCI, percutaneous coronary interventions.
Predictors of primary endpoint and secondary endpoints at univariate and multivariate analysis
| UnivariateP | MultivariateP | AdjustedHR (95%CI) | |
|---|---|---|---|
| Primary endpoints | |||
| Age | .04 | .23 | 1.00 (1.00-1.20) |
| Sex, male | .05 | .79 | 1.23 (0.65-1.41) |
| GFR <60 | .02 | .40 | 1.62 (1.03-2.52) |
| Previous MI | .03 | .50 | 1.54 (0.21-1.90) |
| Ejection fraction | .05 | .12 | 1.00 (1.00-1.64) |
| STS mortality | .04 | .30 | 1.10 (0.73-1.21) |
| NYHA III-IV | .02 | .04 | 0.19 (0.04-0.60) |
| BCIS-JS RI<4 | .01 | .02 | 0.53 (0.19-0.87) |
| Death | |||
| Age | .03 | .39 | 1.34 (1.01-1.63) |
| Sex, male | .05 | .67 | 1.00 (1.50-1.75) |
| GFR <60 | .04 | .37 | 1.21 (1.04-2.02) |
| Previous MI | .05 | .46 | 1.61 (0.64-1.82) |
| Ejection fraction | .04 | .24 | 0.81 (1.10-1.91) |
| STS mortality | .05 | .27 | 1.21 (0.90-1.71) |
| NYHA III-IV | .03 | .06 | 0.9 (0.4-1.0) |
| BCIS-JS RI<4 | .04 | .05 | 0.71 (0.41-0.93) |
| Nonfatal MI | |||
| Age | .05 | .16 | 0.33 (0.12-1.61) |
| Sex, male | .05 | .80 | 1.30 (0.14-1.51) |
| GFR <60 | .04 | .24 | 2.01 (0.60-3.02) |
| Previous MI | .04 | .80 | 1.90 (0.41-2.01) |
| Ejection fraction | .05 | .35 | 1.03 (0.91-1.03) |
| STS mortality | .03 | .25 | 2.01 (0.60-2.61) |
| NYHA III-IV | .04 | .07 | 0.73 (1.01-1.51) |
| BCIS-JS RI<4 | .03 | .06 | 0.50 (0.91-1.82) |
| Nonfatal stroke | |||
| Age | .05 | .20 | 1.00 (1.00-1.91) |
| Sex, male | .04 | .26 | 1.23 (0.84-1.90) |
| GFR <60 | .09 | .97 | 1.41 (1.11-2.54) |
| Previous MI | .08 | - | - |
| Ejection fraction | .05 | .05 | 1.73 (1.10-2.31) |
| STS mortality | .05 | .54 | 1.13 (0.91-2.01) |
| NYHA III-IV | .04 | .08 | 0.75 (0.51-1.81) |
| BCIS-JS RI<4 | .04 | .08 | 1.20 (1.04-4.33) |
| New PCI | |||
| Age | .05 | .32 | 1.51 (1.04-2.12) |
| Sex, male | .09 | - | - |
| GFR <60 | .08 | - | - |
| Previous MI | .07 | - | - |
| Ejection fraction | .05 | .93 | 1.54 (0.92-1.91) |
| STS mortality | .05 | .79 | 1.00 (0.91-2.12) |
| NYHA III-IV | .04 | .32 | 0.91 (0.42-1.94) |
| BCIS-JS RI <4 | .04 | .18 | 1.53 (1.01-3.43) |
AVA, aortic valve area; BCIS-JS RI, British Cardiovascular Intervention Society-Jeopardy Score revascularization index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; MI, myocardial infarction; NYHA, Yew York Heart Association; PCI, percutaneous coronary intervention; PAD, peripheral artery disease; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
In 180 patients with concomitant CAD, 88 patients were underwent coronary PCI before TAVI and 92 patients underwent coronary revascularization prior to TAVI indication, considering all previous coronary revascularizations. No patients underwent a staged PCI after TAVI. In these patients, the extent of myocardial revascularization was graded using the BCIS-JS revascularization-index (RI) (range: 0-1) according to the following formula: (BCIS-JS pre -BCIS-JS post)/BCIS-JS pre.13 The median BCIS-JS revascularization index (RI) was 0.67 [0.7]. Thus, the study population was divided into 3 groups: 223 patients without CAD (no-CAD), 99 patients with extensive revascularization (RI> 0.67, ER-CAD), and 81 patients with less extensive revascularization (RI ≥ 0.67, LER-CAD). The baseline characteristics of patients are listed in table 1 and table 2 of the supplementary data. Patients without CAD and patients with more complete revascularization (ER-CAD, RI> 0.67-1.0) had better MACCE-free survival compared with those with less extensive revascularization (LER-CAD, RI ≤ 0.67; P=.042, figure 1 of the supplementary data). This result was driven by a significant mortality reduction ([SHR, 1.31; 95%CI, 0.97-1.54; P=.041], figure 2 of the supplementary data). Of note, the incidence of nonfatal MI was higher in patients with concomitant CAD independently of the revascularization extent achieved before TAVI compared with no-CAD patients ([SHR, 1.74; 95%CI, 1.28-2.50; P=.046], figure 2 of the supplementary data). In the multivariate analysis, BCIS-JS RI ≤ 0.67 (HR, 2.10; 95%CI, 1.35-5.83; P=.002), BCIS-JS post (HR, 0.17; 95%CI, 0.24-1.34; P=.024) and NYHA class III-IV NYHA class III-IV (HR, 1.19; 95%CI 1.02-1.39; P=.018) were independent predictors of MACCE.
DISCUSSIONThe optimal strategy to manage concomitant CAD in patients with AS who are candidates for TAVI is still under debate.3–7 In the present study we find that a large residual myocardium burden, assessed by BCIS-JS, was associated with a worse clinical outcome, mainly driven by higher mortality. Furthermore, the outcomes of patients with a small extent of jeopardized myocardium before TAVI was similar to that of patients without CAD. Thus, these findings suggest that more extensive revascularization should be achieved in CAD patients before TAVI. When coronary lesions are recognized in patients with AS undergoing TAVI, the safety of the procedure without prior revascularization is unknown. This is because the major trials that led to approval of TAVI required revascularization of significant coronary stenosis in main branch epicardial vessels within 30 days of TAVI.16–18 On the other hand, PCI in AS patients is challenging, as the risk of PCI in this setting is perceived to be high.19 Recently, studies evaluating PCI outcomes in AS have shown that the results can be favorable with careful patient selection.20–24
Of note, not only clinical considerations, but also the amount of jeopardized myocardium and target lesion selection can modulate PCI efficacy. In the present study investigating the clinical impact of the extent of jeopardized myocardium before TAVI, we found that a large amount of jeopardized myocardium before TAVI (BCIS-JS> 4) influenced the clinical impact after TAVI (figure 4). In contrast, a BCI-JS ≤ 4 was associated with a clinical outcome comparable to that of patients without CAD (figure 4). A previous single-center prospective registry including 191 consecutive patients with severe AS referred for TAVI demonstrated that 30-day mortality did not differ significantly between patients with CAD treated by PCI vs no CAD.21 In this study, PCI was performed only for stenosis involving the proximal or mid segments of major coronary arteries but residual myocardial jeopardy prior to TAVI was not assessed. Accordingly, the POL-TAVI registry demonstrated that myocardial revascularization prior TAVI improved 30-day survival to levels comparable to that of patients without obstructive CAD at baseline.25 Surprisingly, neither baseline nor residual Syntax Score values affected outcome.25 In a recent meta-analysis, D’Ascenzo F et al.26 evaluated the impact of CAD severity (assessed as Syntax Score [SS]) and of residual incomplete revascularization (assessed as residual SS [rSS]) on mortality after TAVI. An SS> 22 was associated with increased 1-year mortality and a rSS less than 8 was associated with a lower 1-year risk of death. In contrast, the ACTIVATION (Percutaneous Coronary Intervention Prior to Transcatheter Aortic Valve Implantation) data have recently demonstrated similar rates of death and rehospitalization at 1 year between PCI and no PCI prior to TAVR; however, the noninferiority margin was not met and a subanalysis according to myocardial at risk was not performed.27
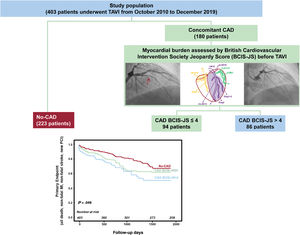
Central illustration. Study design and main results. A total of 403 patients with severe aortic stenosis and concomitant coronary artery disease underwent TAVI were enrolled in the study. The myocardial burden was assessed by BCIS-JS before TAVI. Patients with a lower myocardial burden before TAVI (BCIS-JS<4) had better MACCE-free survival than those with more extensive jeopardized myocardium (BCIS-JS ≥ 4; P=.049). BCIS-JS; British Cardiovascular Intervention Society Jeopardy Score; CAD, coronary artery disease; PCI, percutaneous coronary interventions; TAVI, transcatheter aortic valve implantation.
In the present study, we assessed the extent of jeopardized myocardium by BCIS-JS. The choice was based on the fact that, unlike other angiographic scoring systems focused on lesion-specific characteristics (such as SS), the BCIS-JS is an easy-to-apply classification of the extent of CAD according to myocardial territory at risk.13 This score and its derived RI appear to be the most suitable for the work-up of critical patients.13,28 Of note, we found that a BCIS-JS ≤ 4 before TAVI was associated with better outcomes. This finding indicates that not all lesions have to be revascularized to achieve the clinical advantage. In contrast, this supports the notion that, if TAVI is planned, a minimalistic revascularization plan is not sufficient. In this regard, we also noticed that the improvement in MACCE associated with more extensive revascularization before TAVI was mainly due to the reduction in mortality. Indeed, in our study, as also reported in a previous registry,21 the incidence of MI remained higher in patients with concomitant CAD compared with those without CAD. Although the incidence of MI is higher in CAD patients undergoing to TAVI, independently of coronary revascularization degree, this does not appear to have an impact on hard clinical endpoints such as death.
In the near future, further information is expected to come from important, specifically designed, trials. The ongoing NOTION-3 trial (ClinicalTrials.gov. Identifier: NCT03058627) plans to randomize 452 patients with severe AS and CAD to either fractional flow reserve-guided full revascularization before TAVI in a staged approach, or TAVI alone. The COMPLETE TAVI trial (ClinicalTrials.gov. Identifier: NCT04634240) will randomize 4000 patients referred for TAVI to either angiography-guided PCI after TAVI or medical treatment.
Another important aspect in this scenario is the timing of revascularization. Recently, Kumar A et al.29 found no differences in all-cause mortality or strokes in patients undergoing PCI during or after TAVI compared with patients receiving PCI before TAVI.
LimitationsFirst, although this is a large single-center registry on the subject of the impact of CAD on TAVI outcome with simultaneous evaluation of its complexity and management, the sample was too small to comprehensively address and evaluate the clinical impact. Second, coronary revascularization was performed without a protocol and the extent of coronary revascularization and the use of fractional flow reserve to guide revascularization were left at the operator's discretion. Finally, the safety and efficacy of PCI prior to TAVI compared with isolated TAVI can only be best tested in randomized trials.
CONCLUSIONSRisk factors for AS have been shown to be similar to atherosclerosis. Consequently, CAD is often concurrently found in patients presenting with severe AS. The prognostic role of CAD in patients with severe AS is controversial. It can be considered a detrimental factor or alternatively an innocent bystander marker of high risk. However, in the TAVI era, important unresolved questions are whether, how and when to treat coexisting CAD. Currently, PCI is recommended (IIa) in patients with a coronary artery diameter stenosis> 70% in proximal segments.9 Our results show that a small extent of jeopardized myocardium before TAVI (BCIS-JS ≤ 4) improves prognosis after TAVR. The reduction of CAD burden (assessed by BCIS-JS) before TAVI in patients with more severe CAD could improve prognosis.
These results could apply not only to high-risk patients but especially to most low- and intermediate-risk patients, who are those addressed by emerging indications for TAVI.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSC. Aurigemma and M. B. Giannico are the coprincipal investigators and should be considered cofirst authors.C. Trani, F. Burzotta and C. Aurigemma analyzed and interpreted the data and drafted and revised the final version of the manuscript. M. B. Giannico, S. Cangemi and F. Burzotta performed the evaluation of BCIS-JS. All other authors (P. Bruno, A. M. Leone, F. Bianchini, E. Romagnoli) collected the clinical data and interpreted the data. C. Trani, G. Gaspardone, and F. Crea helped to interpret the data and critically reviewed the manuscript.
CONFLICTS OF INTERESTF. Burzotta, C. Trani and C. Aurigemma received speaker's fees from Abbott, Medtronic, and Abiomed. A. M. Leone. received speaking honoraria from St Jude Medical/Abbott, Medtronic, Abiomed and from Bracco Imaging. P. Bruno received speaker's fees from Abbott. A. Gaspardone received proctorship honoraria from Hearstich e Lifetech. The other authors have no conflicts of interest.
- –
TAVI has revolutionized the treatment of severe AS and the indication for TAVI is expanding to ever-younger and lower-risk patient groups.
- –
Important unresolved questions are whether, how and when to treat coexisting CAD.
- –
Currently, PCI is recommended (IIa) in patients with a coronary artery diameter stenosis> 70% in proximal segments.
- –
Of note, we found that a small CAD burden before TAVI (BCIS-JS≤ 4) was associated with better outcomes.
- –
Our results support the notion that leaving extensive areas of myocardium unrevascularized is associated with an adverse outcome in patients with AS and CAD undergoing TAVI.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.05.020