Some reports have described a change in the etiologic spectrum of constrictive pericarditis. In addition, data on the relationship between its clinical presentation and etiology are lacking. We sought to describe the etiologies of the disease, their relationship with its clinical presentation and surgical findings, and to identify predictors of poor outcome.
MethodsWe analyzed 140 consecutive patients who underwent surgery for constrictive pericarditis over a 34-year period in a single center.
ResultsThe etiology was idiopathic in 76 patients (54%), acute idiopathic pericarditis in 24 patients (17%), tuberculous pericarditis in 15 patients (11%), purulent pericarditis in 10 patients (7%), and cardiac surgery, radiation and uremia in 5, 3 and 2 patients respectively (4%, 2% and 1%). Mean duration of symptoms before pericardiectomy was 19 months (standard deviation, 44 months), the most acute presentation being for purulent pericarditis (26 days [range, 7-60 days]) and the most chronic for idiopathic cases (29 months [range, 4 days-360 months]). Perioperative mortality was 11%. There was no difference in mortality between etiologies. Median follow-up was 12 years (range, 0.1-33.0 years) in which 50 patients died. In a Cox-regression analysis, age at surgery, advanced New York Heart Association functional class (III to IV) and previous acute idiopathic pericarditis were associated with increased mortality during follow-up.
ConclusionsMost cases of constrictive pericarditis are idiopathic. Cardiac surgery and radiation accounted for a minority of cases. Etiologic investigations are warranted only in acute or subacute presentations. Age, advanced functional class, and previous acute idiopathic pericarditis are associated with increased mortality.
Keywords
Since constrictive pericarditis (CP) was first described in 1669 by Lower,1 some clinical aspects may have changed. Some studies2 have recently suggested that the etiologic spectrum has changed in the last decades, basically due to an increase in the number of cases secondary to cardiac surgery (CS) or previous radiotherapy. However, this finding could be misleading. First, these studies were conducted in centers with a high cardiac surgical activity,3 which could introduce some bias in the etiologic spectrum. In addition, the relationship between symptom duration before pericardiectomy and the etiology of CP was not described in detail. Finally, previous studies usually did not include accurate information on the different diagnostic procedures or imaging studies used to diagnose CP.
Our center, a referral center for pericardial diseases, has used a standardized protocol for diagnosis and management of pericardial disease4,5 since 1975. In the present report we describe the etiologic spectrum, the relationship between the chronicity of clinical symptoms and the etiology of CP, the use of the different diagnostic procedures, and predictors of poor outcome, in a series of 140 consecutive patients with CP who underwent surgery in our institution in the last 34 years.
METHODSWe conducted a retrospective study in a single tertiary center. The study was approved by the ethics committee of our institution. A total of 140 patients underwent pericardiectomy in our institution (Hospital Universitari Vall d’Hebron, Barcelona, Spain) for confirmed CP between January 1978 and May 2012. The clinical records were individually reviewed by a single investigator (Andreu Porta-Sánchez), devoting special attention to the presence of inflammatory systemic diseases, previous episodes of acute pericardial diseases, thoracic or mediastinal irradiation or previous CS, and the clinical and imaging studies performed. The time elapsed from the potential causative illness to the onset of symptoms of CP was quantified as well as the time to pericardiectomy. Surgical features were also recorded, with special attention to the involvement of the parietal and visceral layers. Mortality and follow-up data were obtained through outpatient clinic visits or by the electronic clinical records or by phone. Data from the Spanish National Institute of Statistics6 were obtained to generate an age and sex-matched population, and an estimated Kaplan Meier survival curve was generated to compare the long-term overall mortality in our patients with the general population.
DefinitionsIdiopathic CP was diagnosed when no apparent etiology was identified. Postacute idiopathic pericarditis was defined as CP occurring after a well-defined episode of acute idiopathic pericarditis (typical chest pain, pericardial friction rub, serial changes in the ST segment of the electrocardiogram. Tuberculous CP was defined when Koch bacillus was isolated from a pericardial specimen or when caseous granulomas were identified in a biopsy specimen. Purulent CP was diagnosed when pericardial fluid was macroscopically purulent or had a high polymorphonuclear content (> 90%). Postsurgical CP was diagnosed when CP was developed after an open-heart surgery. Radiotherapy CP was defined by the development of CP after a therapeutic chest radiation and after the exclusion of other causes of CP. In an arbitrary manner, the terms acute, subacute and chronic CP were applied, respectively, to patients who required pericardiectomy before 3 months, between 3 and 6 months, or after 6 months of the onset of clinical symptoms. Pericardial calcification was defined as the presence of calcification on a chest X-ray. Left ventricular systolic function was considered abnormal if left ventricular ejection fraction was estimated below 50%. The following echo-Doppler findings were considered diagnostic: the presence of pericardial thickening and notch of the interventricular septum, changes in E velocity superior to 25% related to respiratory movements, a rapid E wave deceleration, suprahepatic vein flow with “W” morphology or predominance of the diastolic component with diastolic inversion during expiration. The amount of pericardial effusion (PE) was classified as mild (< 10mm of echo-free space in anterior and posterior pericardial spaces), moderate (10-20mm) or severe (> 20mm). Additional imaging studies were performed to confirm pericardial thickening or calcification (computed tomography [CT]) or to exclude an infiltrative cardiomyopathy (magnetic resonance imaging). If diagnosis of CP was still unclear after the imaging studies, a right and left heart catheterization was performed. Pericardiectomy was performed through median sternotomy in all cases. Although there have been several surgical advances in the last 30 years, the surgical approach for the treatment of CP has not substantially changed over this period. A wide pericardiectomy was attempted (phrenic-to-phrenic and diaphragmatic pericardium) and was successfully achieved in 95% of cases. Perioperative mortality was defined as death occurring during surgery or within the first 30 days of hospitalization.
Statistical AnalysisThe 1-way analysis of variance test was used for comparisons of continuous variables between etiologic groups. The chi-square test was used to compare the frequencies of qualitative variables. A bivariate Cox regression model was used to identify potential prognostic factors and multivariate Cox regression analysis to identify the independent predictors of mortality. Variables with a P value < .2 in the bivariate Cox regression analyses were included in a stepwise Cox regression model. The discriminatory power of the final model was assessed by means of C-statistics, which were estimated by bootstrapping, taking 1000 sample bootstraps. Proportional hazards assumption was graphically checked for each variable. SPSS (IBM Corp., Released 2010, IBM SPSS Statistics for Windows, Version 19.0; Armonk, New York, United States) was used for the statistical analysis.
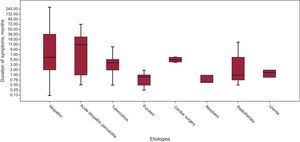
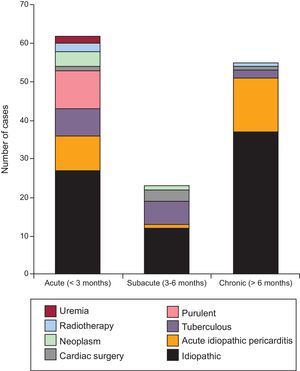
RESULTSClinical FindingsTable 1 summarizes the demographics and clinical findings of our 140 patients, as well as the etiologies of CP. The most common etiology was idiopathic (54% of cases). Inflammatory/infectious pericarditis (acute idiopathic/viral pericarditis, tuberculous and purulent pericarditis) accounted for 35% of cases. All patients presenting with acute or subacute pericarditis were treated with anti-inflammatory drugs, independently of the suspected etiology. Of 140 patients, 37 (26.4%) had had a previous episode of acute pericarditis. Previous acute pericarditis was more frequent in the idiopathic CP group (59.5%) than in the identifiable etiology CP group (40.5%). Remarkably, previous CS and radiotherapy accounted for only 4% and 2% of cases, respectively. The symptom duration for each etiology is summarized in Figure 1. Overall mean time of symptoms was 19.5 months (ranging from 8 days to 360 months). Figure 2 shows the distribution by groups (acute, subacute, or chronic) of the different etiologies of CP and their relationship to symptom duration before the pericardiectomy. Interestingly, almost all patients with purulent, tuberculous and neoplastic CP had an acute or subacute presentation, the most acute illness being observed in purulent CP, with a mean symptom duration of 0.9 months. In contrast, most cases of chronic illness were due to an idiopathic CP or postacute idiopathic pericarditis CP.
Demographics and Clinical Manifestations and Frequencies of Etiologies
| Demographics | |||
| Sex, male | 99 (70.7) | ||
| Mean age at surgery, y | 54.6 (17-80) | ||
| Diabetes mellitus | 16 (11.4) | ||
| Hypertension | 28 (20.0) | ||
| Renal impairment | 14 (10.0) | ||
| Clinical findings | |||
| Congestive heart failure | 124 (87.3) | ||
| Dysponea | 117 (82.4) | ||
| Pre-surgery NYHA functional class | |||
| I | 26 (18.6) | ||
| II | 65 (46.4) | ||
| III | 34 (24.3) | ||
| IV | 15 (10.7) | ||
| Chest pain | 46 (32.6) | ||
| Fever | 33 (23.4) | ||
| Syncope | 2 (1.4) | ||
| Etiology | |||
| Idiopathic | 32 (54.2)a | 44 (54.3)b | 76 (54.3)c |
| Acute idiopathic pericarditis | 8 (13.6)a | 16 (19.7)b | 24 (17.1)c |
| Tuberculous | 8 (13.6)a | 7 (8.6)b | 15 (10.7)c |
| Purulent | 3 (5.1)a | 7 (8.6)b | 10 (7.1)c |
| Neoplastic | 4 (6.8)a | 1 (1.2)b | 5 (3.6)c |
| Cardiac surgery | 1 (1.7)a | 4 (4.9)b | 5 (3.6)c |
| Radiotherapy | 3 (5.1)a | 0 (0.0)b | 3 (2.1)c |
| Uremia | 0 (0.0)a | 2 (2.5)b | 2 (1.4)c |
| Total | 59 (42.1)a | 81 (57.9)b | 140 (100.0)c |
NYHA, New York Heart Association.
Data are expressed as No. (%) or median (range).
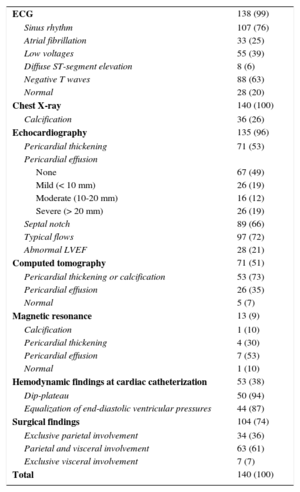
Table 2 shows the imaging and invasive tests performed and their diagnostic yield. Calcification in chest X-ray was seen in 26% of patients. Pericardial effusion was more often present in patients with an acute or subacute presentation compared with those with chronic presentation (48% and 35% vs 9% respectively; P < .001). Echocardiography showed diagnostic findings of constriction and/or pericardial thickening in 113 patients (83.7%). Moderate to severe PE was seen in 43 patients (30.7%) during their clinical course. The proportion of significant PE differed among etiologies: 3 etiologies had an important proportion of patients with significant PE—tuberculous (66.7%), radiotherapy (66%), neoplasm (60%), uremia (50%) and purulent (50%)—while the proportion of patients with significant PE was lower in those with postsurgery CP, postacute idiopathic pericarditis, and idiopathic CP (40.0%, 29.2% and 17.1%, respectively). Computed tomography, performed in 71 patients (50%), showed calcification or pericardial thickening (mean [standard deviation] pericardial thickness of 11.4 [6.8] mm) or PE in 93% of patients, and was normal in only 5 patients. The strength of CT in patients with suspected CP is its high positive predictive value for detecting calcification or thickening of the pericardium. Cardiac catheterization was performed in 53 patients (38%) and showed typical findings of constriction (equalization of end-diastolic pressures with dip-plateau morphology of diastolic ventricular curves and findings of ventricular interdependence) in 50 of them (95%), with a mean (standard deviation) right ventricular telediastolic pressure of 18.8, (5.1) mmHg and a mean (standard deviation) left ventricular telediastolic pressure of 20.8, (5.0) mmHg.
Diagnostic Tests
| ECG | 138 (99) |
| Sinus rhythm | 107 (76) |
| Atrial fibrillation | 33 (25) |
| Low voltages | 55 (39) |
| Diffuse ST-segment elevation | 8 (6) |
| Negative T waves | 88 (63) |
| Normal | 28 (20) |
| Chest X-ray | 140 (100) |
| Calcification | 36 (26) |
| Echocardiography | 135 (96) |
| Pericardial thickening | 71 (53) |
| Pericardial effusion | |
| None | 67 (49) |
| Mild (< 10 mm) | 26 (19) |
| Moderate (10-20 mm) | 16 (12) |
| Severe (> 20 mm) | 26 (19) |
| Septal notch | 89 (66) |
| Typical flows | 97 (72) |
| Abnormal LVEF | 28 (21) |
| Computed tomography | 71 (51) |
| Pericardial thickening or calcification | 53 (73) |
| Pericardial effusion | 26 (35) |
| Normal | 5 (7) |
| Magnetic resonance | 13 (9) |
| Calcification | 1 (10) |
| Pericardial thickening | 4 (30) |
| Pericardial effusion | 7 (53) |
| Normal | 1 (10) |
| Hemodynamic findings at cardiac catheterization | 53 (38) |
| Dip-plateau | 50 (94) |
| Equalization of end-diastolic ventricular pressures | 44 (87) |
| Surgical findings | 104 (74) |
| Exclusive parietal involvement | 34 (36) |
| Parietal and visceral involvement | 63 (61) |
| Exclusive visceral involvement | 7 (7) |
| Total | 140 (100) |
ECG, electrocardiogram; LVEF, left ventricular ejection fraction.
Data are expressed as No. (%).
Surgery showed involvement of both layers of the pericardium in most patients (61%) whereas an exclusive visceral involvement was only seen in 5 patients (7%).
Microscopic PathologyData on surgical pathology was available for 120 patients. Most of the patients had a fibrinous tissue (95 patients, 79%), while lymphoplasmacytic inflammation was identified in 5 patients (5.8%). Granulomatous inflammation was seen in 10 patients (8.3%) and in 4 of them Mycobacterium. tuberculosis was identified in the biopsy. Four patients (3%) had adenocarcinoma identified in the biopsy. Three patients (2.5%) had an acute inflammation with neutrophils and lymphocytes. Only 1 patient had findings consistent with a purulent pericarditis.
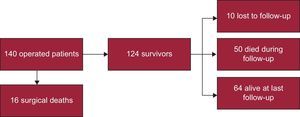
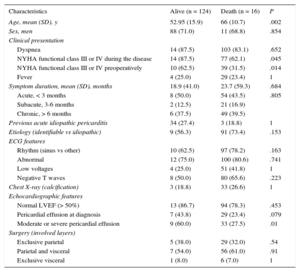
Perioperative MortalityFigure 3 shows the flow-chart of patients. Perioperative mortality was 11.4% (16 out of 140 patients). Death was due to low-cardiac output syndrome in 6 patients, complications of a prolonged intensive care unit stay ending in sepsis in 5, mixed cardiogenic and septic shock in 2, advanced cancer in 1, and stroke in 1. In 1 patient the cause of death could not be determined. Regarding etiologies, 8 of the 16 patients who died in the first month had idiopathic CP, 2 patients had post-CS CP, 2 had neoplasic CP, 2 had purulent CP, 1 patient had a postacute idiopathic pericarditis CP, and 1 patient had radiation induced CP. Table 3 shows the differences between patients who died and those who survived. Perioperative mortality was associated with older age (66.1 years vs 52.9 years; P < .002), advanced New York Heart Association functional class (III or IV) at the time of surgery (odds ratio = 3.63; 95% confidence interval [95%CI], 1.23-10.70), and with the presence of moderate or severe PE during the course of the disease (odds ratio = 3.40; 95%CI, 1.20-9.91]). There was a trend towards a higher mortality in the acute presentation (12.9% in acute patients vs 8.7% in subacute vs 10.9% in chronic). There were no differences in perioperative mortality between etiologies.
Baseline Characteristics of Patients With Postoperative Mortality*
| Characteristics | Alive (n = 124) | Death (n = 16) | P |
|---|---|---|---|
| Age, mean (SD), y | 52.95 (15.9) | 66 (10.7) | .002 |
| Sex, men | 88 (71.0) | 11 (68.8) | .854 |
| Clinical presentation | |||
| Dyspnea | 14 (87.5) | 103 (83.1) | .652 |
| NYHA functional class III or IV during the disease | 14 (87.5) | 77 (62.1) | .045 |
| NYHA functional class III or IV preoperatively | 10 (62.5) | 39 (31.5) | .014 |
| Fever | 4 (25.0) | 29 (23.4) | 1 |
| Symptom duration, mean (SD), months | 18.9 (41.0) | 23.7 (59.3) | .684 |
| Acute, < 3 months | 8 (50.0) | 54 (43.5) | .805 |
| Subacute, 3-6 months | 2 (12.5) | 21 (16.9) | |
| Chronic, > 6 months | 6 (37.5) | 49 (39.5) | |
| Previous acute idiopathic pericarditis | 34 (27.4) | 3 (18.8) | 1 |
| Etiology (identifiable vs idiopathic) | 9 (56.3) | 91 (73.4) | .153 |
| ECG features | |||
| Rhythm (sinus vs other) | 10 (62.5) | 97 (78.2) | .163 |
| Abnormal | 12 (75.0) | 100 (80.6) | .741 |
| Low voltages | 4 (25.0) | 51 (41.8) | 1 |
| Negative T waves | 8 (50.0) | 80 (65.6) | .223 |
| Chest X-ray (calcification) | 3 (18.8) | 33 (26.6) | 1 |
| Echocardiographic features | |||
| Normal LVEF (> 50%) | 13 (86.7) | 94 (78.3) | .453 |
| Pericardial effusion at diagnosis | 7 (43.8) | 29 (23.4) | .079 |
| Moderate or severe pericardial effusion | 9 (60.0) | 33 (27.5) | .01 |
| Surgery (involved layers) | |||
| Exclusive parietal | 5 (38.0) | 29 (32.0) | .54 |
| Parietal and visceral | 7 (54.0) | 56 (61.0) | .91 |
| Exclusive visceral | 1 (8.0) | 6 (7.0) | 1 |
ECG, electrocardiogram, LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
Data are expressed as No. (%) or mean (standard deviation).
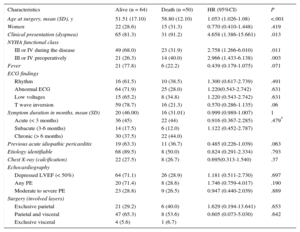
Ten patients were lost to follow-up after hospital discharge. Median follow-up among survivors was 12 years [range, 0.1-33.0 years]. Figure 4 shows the Kaplan Meier curve of overall survival for our cohort of patients and an age and sex-matched Spanish population. Mean survival was 220 months (95%CI, 196-245). At 20 years follow up, 50.9% of patients were still alive. Compared with the general age and sex-matched Spanish population, the survival rate of these patients was markedly inferior, although curves trended to overlap beyond 20 years of follow up. There were 50 deaths after the postoperative period (30 days). There were no differences in mortality between different etiologies. The bivariate-Cox model analysis of patients who survived the perioperative period and completed the follow-up (114 patients) identified age (hazard ratio [HR]= 1.05; P < .001), the presence of dyspnea at clinical presentation (HR = 4.66; P = .013), and New York Heart Association functional class (III-IV) during the disease or before surgery (HR = 2.76; P = .011 and 2.97, P = .003 respectively) as potential predictors of late mortality (Table 4). The multivariate analysis retained age at surgery (HR = 1.052; 95%CI, 1.017-1.088; P = .003), advanced New York Heart Association functional class before surgery (HR = 4.028; 95%CI, 1.792-9.054; P = .001), and having had a previous episode of acute pericarditis (HR = 2.931; 95%CI, 1.261-6.810; P = .012) as long-term mortality predictors of death (Table 5). The C-statistic of our final model was 0.75, thus indicating an adequate discriminatory power.
Univariate Cox Regression Estimates of Baseline Clinical Characteristics
| Characteristics | Alive (n = 64) | Death (n =50) | HR (95%CI) | P |
|---|---|---|---|---|
| Age at surgery, mean (SD), y | 51.51 (17.10) | 58.80 (12.10) | 1.053 (1.026-1.08) | <.001 |
| Women | 22 (28.6) | 15 (31.3) | 0.770 (0.410-1.448) | .419 |
| Clinical presentation (dyspnea) | 65 (81.3) | 31 (91.2) | 4.658 (1.386-15.661) | .013 |
| NYHA functional class | ||||
| III or IV during the disease | 49 (68.0) | 23 (31.9) | 2.758 (1.266-6.010) | .011 |
| III or IV preoperatively | 21 (26.3) | 14 (40.0) | 2.966 (1.433-6.138) | .003 |
| Fever | 21 (77.8) | 6 (22.2) | 0.439 (0.179-1.075) | .071 |
| ECG findings | ||||
| Rhythm | 16 (61.5) | 10 (38.5) | 1.300 (0.617-2.739) | .491 |
| Abnormal ECG | 64 (71.9) | 25 (28.0) | 1.220(0.543-2.742) | .631 |
| Low voltages | 15 (65.2) | 8 (34.8) | 1.220 (0.543-2.742) | .631 |
| T wave inversion | 59 (78.7) | 16 (21.3) | 0.570 (0.286-1.135) | .06 |
| Symptom duration in months, mean (SD) | 20 (46.00) | 16 (31.01) | 0.999 (0.989-1.007) | 1 |
| Acute (< 3 months) | 36 (45) | 22 (44) | 0.916 (0.367-2.285) | .479* |
| Subacute (3-6 months) | 14 (17.5) | 6 (12.0) | 1.122 (0.452-2.787) | |
| Chronic (> 6 months) | 30 (37.5) | 22 (44.0) | ||
| Previous acute idiopathic pericarditis | 19 (63.3) | 11 (36.7) | 0.485 (0.226-1.039) | .063 |
| Etiology identifiable | 68 (89.5) | 8 (50.0) | 0.824 (0.291-2.334) | .793 |
| Chest X-ray (calcification) | 22 (27.5) | 8 (26.7) | 0.695(0.313-1.540) | .37 |
| Echocardiography | ||||
| Depressed LVEF (< 50%) | 64 (71.1) | 26 (28.9) | 1.181 (0.511-2.730) | .697 |
| Any PE | 20 (71.4) | 8 (28.6) | 1.746 (0.759-4.017) | .190 |
| Moderate to severe PE | 23 (28.8) | 9 (26.5) | 0.947 (0.440-2.039) | .889 |
| Surgery (involved layers) | ||||
| Exclusive parietal | 21 (29.2) | 6 (40.0) | 1.629 (0.194-13.641) | .653 |
| Parietal and visceral | 47 (65.3) | 8 (53.6) | 0.605 (0.073-5.030) | .642 |
| Exclusive visceral | 4 (5.6) | 1 (6.7) | ||
95%CI, 95% confidence interval; ECG, electrocardiogram, HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PE, pericardial effusion; SD, standard deviation.
Baseline characteristics of patients who survived the first month after surgery and completed the long-term follow-up (114 patients).
Unless otherwise indicated, data are expressed as No. (%), mean (standard deviation).
Adjusted Independent Predictors of Adverse Outcome Adjusted for Potential Confounders
| Variable | P | HR (95%CI) |
|---|---|---|
| Age at surgery | .002 | 1.054 (1.020-1.090) |
| NYHA functional class III or IV preoperatively | .006 | 3.139 (1.397-7.055) |
| Previous acute idiopathic pericarditis | .010 | 2.790 (1.274-6.113) |
| Negative T waves in ECG | .299 | 0.682 (0.331-1.404) |
95%CI, 95% confidence interval; ECG, electrocardiogram; HR, hazard ratio; NYHA, New York Heart Association.
This is a contemporary series of a large group of patients with CP who underwent pericardiectomy in a single institution serving as referral center for pericardial diseases and using a standardized protocol for its diagnosis and management followed for more than 30 years. The analysis of this series provides new insights into the etiologic spectrum, clinical presentation, usefulness of diagnostic methods and prognostic factors in this disease. By contrast to prior contemporary reports, our study shows that most CP are idiopathic, with CS and radiation accounting for only a minority of cases. Long-term survival after pericardiectomy is not affected by the etiology, while age, advanced functional class, and previous acute idiopathic pericarditis are potential prognostic factors.
Etiologic SpectrumBased on series of patients who underwent surgery between 1970 and 2000, some authors2,3,7–9 have suggested a change in the etiologic spectrum of CP with a trend toward a larger proportion of patients with postcardiac surgery (18% to 37% of cases) and postradiation CP (9% to 31% of cases). In particular, CS accounted for a proportion as high as 37% in one series.3 By contrast, in our series, the most frequent etiologies were idiopathic (54% of cases) followed by inflammatory/infectious CP (35%), whereas postsurgical and postradiation CP accounted for only 4% and 2% of cases, respectively. No changes between the frequencies of etiologies were observed between the periods 1978-1995 and 1996-2012. Although this discrepancy may partially be explained by the different structure of hospitals, it seems quite improbable that a different volume in CS, oncology and radiotherapy procedures could lead to such large differences on estimates. According to the incidence of CP after CS (0.09%–0.30%) reported in different studies,2,3 we should have seen a larger number of cases. Another plausible explanation may be that CP after CS is often transient.10,11 Thus one can postulate that some pericardiectomies could have been avoided if a more conservative strategy had been adopted.12 It is our practice to be specially vigilant for this possible outcome13 and establish the indication of pericardiectomy only in cases with a severe and persistent constriction.
Remarkably, in our study an acute viral/idiopathic pericarditis was the cause of CP in 17% of cases. This is in accordance with prospective studies4,14 that have estimated the probability of developing CP after an acute pericarditis to be as low as 1%.
Relationship Between Etiology and Clinical Features. Considerations for Etiologic StudiesConstrictive pericarditis is the final manifestation of a very wide range of pericardial insults. The time elapsed between the causal illness and the development of constriction can be variable. The relationship between the etiology and the clinical development of constriction has not been analyzed in previous studies. This information is important in 2 ways: on the one hand, in the attempt to predict the possible chronology of progression to constriction after the different kinds of aggression to the pericardium, and on the other hand, to establish the advisability of etiologic investigations in patients with constriction. For instance, nearly all cases of constriction due to specific and potentially treatable causes (mainly purulent or tuberculous pericarditis) require pericardiectomy in the first 6 months (acute and subacute constriction), an observation that is in accordance with our previous experience with tuberculous and purulent pericarditis.15–17 In particular, purulent pericarditis can show a hyperacute progression to constriction, the shortest interval being 8 days. Accordingly, patients with purulent pericarditis must be carefully monitored in the first weeks and patients with tuberculous pericarditis in the first months. If constriction does not develop in the first 6 months, it is unlikely to appear later on. In contrast to acute and subacute cases, no specific treatable etiologies were found in the group of patients with chronic CP (more than 6 months). These findings suggest that, in patients who present with CP, etiologic investigations are only warranted for acute and subacute cases.
Use and Diagnostic Yield of Imaging and Diagnostic TechniquesIn our series, calcification of the pericardium in the chest X-ray was seen only in 26% of cases, in agreement with other recent series.18 However, this finding should be carefully searched for, as its presence in a patient with clinical suspicion of CP is sufficient to establish a firm diagnosis. The global diagnostic yield of echocardiography-Doppler was approximately 84%. An unsuspected finding was the high percentage of patients with PE, which can be explained by the fact that 43% of patients underwent surgery during the first 3 months of the disease when patients remained symptomatic despite medical treatment (anti-inflammatory drugs and/or diuretics when indicated). These patients could have had an effusive-CP.19 The diagnostic yield of CT was as high as 93% and only 5 patients (7%) had a normal CT. Thus, although cases of CP with normal pericardial thickness have been described,20 a strictly normal CT is evidence against a diagnosis of CP. However, this finding could be biased as the possibility that the diagnosis of CP could have been missed in some patients with normal CT cannot be excluded. Despite this high diagnostic yield, we believe that catheterization is not necessary in most patients. The diagnostic yield of magnetic resonance cannot be established from our data, as it was only performed in 9% (13 of 140 patients). In fact, CT may be superior to magnetic resonance due to its greater reliability in detecting calcium. Cardiac catheterization was done in 38% (53 of 140 patients) and showed typical diagnostic findings (dip-plateau with equalization of diastolic ventricular pressure curves in 94% and 87% respectively). Cardiac catheterization was also useful in excluding coronary artery disease in patients with risk factors or with anginal symptoms. During the time-span our series was collected, several advances have been made in imaging techniques and there is a tendency toward a more judicious use of the invasive diagnostic tests in our patients.21 When there is clinical suspicion of CP, echocardiography-Doppler (showing a restrictive physiology) and CT (showing a thickened and/or calcified pericardium) should be enough to establish the diagnosis.
Prognostic FactorsThe perioperative mortality found in our series (11.4%) is in agreement with the experience of other authors.7,22,23 Similar to other series, age and advanced New York Heart Association class were predictors of mortality. Unexpectedly, we also found the presence of PE to be more frequent in patients with a poor surgical outcome. Mortality was similar in acute, subacute and chronic presentations, although there seems to be a trend to higher mortality in the acute presentation. In contrast with the experience of other authors,18 we did not find that the presence of pericardial calcification was as an independent predictor of perioperative mortality.
LimitationsOur study has several limitations mainly related to its retrospective design and to the fact that we only analyzed patients who underwent surgery, thus excluding all patients who were not candidates for surgery because of comorbidities or poor clinical status. A small number of diagnosis of CP could have been missed, which could introduce some bias in the diagnostic yield of the different diagnostic procedures. However, the impact of these limitations is not expected to be sufficiently high to compromise the validity of the conclusions of the study.
CONCLUSIONSIn our experience, idiopathic and postinflammatory/infectious pericarditis are still the most frequent causes of CP leading to pericardiectomy, while CS and radiotherapy-related pericarditis account for only a minority of cases. Purulent, tuberculous and neoplastic pericarditis show a characteristic pattern of acute or subacute progression to constriction. There are no differences in mortality between different etiologies. Echocardiography was the most common technique used to identify a physiologic pattern of restriction/constriction and CT was the most reliable technique to document a thickened or calcified pericardium. Predictors of poor surgical or long-term prognosis are age, advanced clinical status, and the presence of moderate or severe PE.
FUNDINGThis work has been partially supported by RECAVA (Red Temática de Investigación Cooperativa en Enfermedades Cardiovasculares) from RETICS (Redes Temáticas de Investigación Cooperativa en Salud) of the Instituto de Salud Carlos III from the Spanish Ministry of Science and Innovation.
CONFLICTS OF INTERESTNone declared.