Keywords
WHAT IS A CONGENITAL CORONARY ARTERY ANOMALY?
There is an ongoing debate as to what should be considered normal and what should be considered a coronary artery anomaly (CAA). According to Angelini,1 CAA are diagnosed by exclusion, which means that, in those cases in which the coronary artery morphology is not normal, defining normality according to a statistical criteria (interval between plus or minus 2 standard deviations of the mean value). Thus, a CAA would be that coronary artery morphology observed in less than one percent of the general population.1,2 CAA can be produced during normal or pathological cardiogenesis: in the first case, the result would be isolated CAA, on which we focus in this article, whereas, in the second case, they would be associated with other cardiac malformations.
CLASSIFICATION
There are several classifications.1-3 A new one which attempts to standardize diagnostic criteria and groups CAA according to 7 categories has recently been proposed4 (Table 1).
International groups are calling for registries including at least those anomalies that cause the majority of the clinical events (sudden death and myocardial ischemia) attributed to CAA,5-7 constituted by those originating in the contralateral or "wrong" sinus of Valsalva. This group includes single vessel coronary arteries ("hyperdominance" in the classification of Rigatelli et al4), since they have to supply blood to the myocardium arising from a single coronary ostium and establish routes that occasionally are similar to those of the CAA with origin in the wrong sinus of Valsalva (Figure 1).
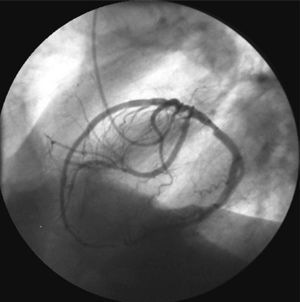
Figure 1. Coronary arteriografía in left anterior oblique projection showing a single vessel right coronary artery.
INCIDENCE
The different definitions or classifications and the analysis of both angiographic8,9 and autopsy series10 and, in recent years, echocardiographic series11 result in a reported incidence of CAA that ranges between 0.1% and 8.4%,1,8,11 making it difficult to establish the exact magnitude of the problem they represent. Overall, the incidence may be quite similar to that of other diseases that we have been able to define more accurately, such
as hypertrophic cardiomyopathy (0.2%)12 or Wolff-Parkinson-White syndrome (0.1% to 0.3%).13
The incidence of anomalous coronary arteries with origin in the wrong sinus and single vessel coronary arteries in coronary angiographic studies ranges between 0.28% and 1.74%.2,3,8,9,14-16 Given their clinical importance, we are going to focus our review on these anomalies.
CLINICAL PRESENTATIONS
Initially, these anomalies were diagnosed during coronary angiographic performed in patients with valve disease or ischemic heart disease, and were considered to have no clinical significance. Later on, there began to be reports of sudden death in young athletes in whom the only sign of disease was an anomaly with origin in the coronary arteries.8,17
The clinical spectrum at presentation is variable: whereas some patients are asymptomatic, others present angina, dyspnea, syncope, acute myocardial infarction, heart failure and sudden death.1,2,5,8,17-20 Currenthy, CAA are considered to be the second most common cause of sudden death in athletes in the United States.21
Does this mean that all CAA should be considered to indicate high risk? Absolutely not, but from a strategic point of view, they should be considered "potentially malignant" until additional tests are performed to rule out myocardial ischemia provoked by the anomaly.
The major concern lies in determining which CAA can present with sudden death. Today, we know that those that follow an interarterial (or intramural) course, those in which the anomalous coronary artery is the dominant one and those that produce clinical signs in patients of less than 30 or 35 years of age are those in which the incidence of sudden death is highest.22-26
For this reason, our approach to a CAA should differ depending on the age of the patient. In young people, under 35 years of age, we will "focus" on preventing sudden death, whereas in those over 35 years of age, the major objective will be to treat myocardial ischemia (Figure 2).
Figure 2. Different approach according to patient age.
Occasionally, because of their origin and anomalous course, CAA can be damaged during surgery for valve replacement, but this can also occur during percutaneous foramen ovale closure. For this reason, the presence of CAA should be ruled out prior to interventions of this type.27-30
PATHOPHYSIOLOGY OF ISCHEMIA IN CORONARY ANOMALIES
There are a number of theories31 to explain the mechanisms that produce ischemia in CAA, but none of them has been clearly demonstrated. In one, the cause is considered to be the marked angulation of the anomalous artery where it emerges from the aorta. In contrast to a normal coronary artery, which is perpendicular to the aorta at its origin, the anomalous coronary artery has to bend over itself to reach, from the opposite sinus of Valsalva, its normal supply territory. Because of this, the ostium of the anomalous coronary artery would be smaller, with valve-like ridges, as compared to the normal circular ostium, and could become compressed in the case of a marked expansion of the aorta, such as that observed during strenuous exercise.32
Another theory refers to the initial course of the CAA. When it is interarterial, between the aorta and pulmonary artery, the increase in the pressure in the 2 vessels, occurring during exertion, would produce a compression of the anomalous coronary artery.33 Moreover, if the initial pathway is intramural, the obstruction may be enhanced since the coronary artery can become deformed within the aortic wall during periods of hypertension.34
Some authors consider that the mechanism leadingtoischemia involves the production of a spasm in the anomalous coronary artery as a result of endothelial damage produced by the anomalous pathway,35 and others, that an intussusception of the proximal portion of the CAA in the aortic wall would be produced.31
Finally, all the mechanisms discussed can produce acute or chronic ischemia (minor ischemic events) that provoke myocardial fibrosis which, in turn, could be the cause of the generation of lethal arrhythmias.36
HOW IS A CORONARY ANOMALY DIAGNOSED?
The diagnosis requires a high index of suspicion and the assessment of its presence under certain circumstances. As we mentioned above, most CAA are discovered incidentally during coronary angiographies performed in patients with ischemic heart disease or valve disease undergoing this study prior to surgery for valve replacement.
Young people who present no coronary risk factors or associated valve disease represent the greatest diagnostic challenge. We should suspect a CAA in the presence of exertional syncope, dyspnea or chest pain indicative of angina.24,26,35 Evidently, the first diagnostic test that we all perform is standard ergometry. However, this has not been shown to be very useful in thesetype of patients since it is usually negative, a fact that indicates that ischemia due to CAA presents only occasionally.35,36
Thus, given that "functional" tests are not of much help, we have to opt for an imaging study which enables us to examine the coronary anatomy. The noninvasive test initially recommended is transthoracic echocardiography (TTE).7,26,37-40 In the majority of these patients, the performance of simple TTE in the parasternal short-axis projection in the plane of the aortic root makes it possible to distinguish the two coronary ostia and even to determine the initial course of the CAA. Pellicia et al studied 1360 elite athletes by means of echocardiography, visualizing the ostium and the proximal portion of left main coronary artery in 97% of the cases and right main coronary artery in 80%.41
Recently, Frommelt et al34 performed echocardiography in all children and adolescents (age range, 3 months to 20 years) referred to their hospital (1997-2002) with symptoms of myocardial ischemia, suspected congenital heart disease or musculoskeletal pain. They all underwent conventional TTE with the aid of Doppler color flow mapping to determine the direction of blood flow. They identified 10 patients with anomalous origin of a coronary artery in the wrong sinus (6 patients with left coronary artery originating in right sinus of Valsalva and 4 with right coronary artery originating in left sinus of Valsalva). Using TTE with color Doppler techniques, they observed an intramural course within the aortic wall in nine of the patients and an intramyocardial course in the remaining patient. When the course of a CAA originating in the wrong sinus is intramural, it may appear to emerge from its normal ostium. Thus, the authors recommend the utilization of color Doppler to determine the flow direction when it is necessary to rule out the presence of an anomaly.
Improvements in echocardiographic imaging techniques enable us to determine the origin and follow the initial course of a CAA in certain groups of patients. Studies have been carried out in children, adolescents and elite athletes, but large series involving the normal adult population have yet to be studied. Thus, the identification of the coronary ostia in young patients with this symptomatology should be carried out systematically in the TTE examination.7,26
In patients with a poor echocardiographic window, or when diagnostic doubt persists despite the clinical indications, to enable the visualization of the origin and initial course of the CAA, transesophageal echocardiography (TEE),7,26,42,43 computed tomography (CT)26,44-46--with electron beam or multislice--(Figure 3) or cardiac magnetic resonance imaging (CMRI)26,47,48 can be performed (Figure 4).
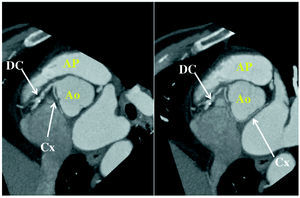
Figure 3. Multidetector computed tomography image showing circumflex coronary artery (Cx) originating in right coronary artery (CD), following a retroaortic course to arrive at its normal distribution territory. Ao indicates aorta; AP, pulmonary artery. Courtesy of Dr. Gabriel C. Fernández, Servicio de Radiología, Hospital Povisa, Vigo, Spain.
Figure 4. Diagnostic protocol proposed in patients under 35 years of age with suspected coronary artery anomaly. CMRI indicates cardiac magnetic resonance imaging; CT, computed tomography; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Which technique should we use and in what order? Each medical team should respond to this question in accordance with the available techniques and their experience. What we should do is identify the CAA and its initial course by means of the least invasive technique available to us. Although TEE has been utilized in the diagnosis and identification of the initial course of some CAA, we consider that, given its semi-invasive nature, it should be employed only after other techniques have failed. Today, owing to the increasing availability and the excellent three-dimensional images that can be obtained, it would appear to be prudent to opt for multidetector CT (Figure 5) or for CMRI.49,50 standard coronary arteriography would be indicated if the other tests do not result in a definitive diagnosis. In this respect, the 36th Bethesda Conference51 for the selection of competitive athletes with cardiovascular anomalies published in April 2005 recommended: "Coronary anomalies should be considered in athletes with exertional syncope or symptomatic ventricular arrhythmia and should be investigated using appropriate studies such as echocardiography, CMR or ultrafast computed tomography imaging. Coronary angiography is indicated if other studies are not diagnostic." In brief, they do not opt for 1 diagnostic study or another.
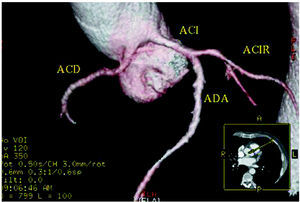
Figure 5. Multislice computed tomography (16 slices); three-dimensional rendered reconstruction showing right coronary artery (ACD) originating in left sinus of Valsalva. ACI indiactes left coronary artery; ACIR, circumflex coronary artery; ADA, anterior descending coronary artery. Courtesy of Dr. Joaquín Alonso, Servicio de Cardiología y Servicio de Diagnóstico por Imagen, Hospital de Fuenlabrada, Madrid, Spain.
In patients over 35 years of age who present with clinical signs of angina or repeated syncope, after the functional test (ergometry, exercise echocardiography, etc), a catheterization is usually performed since the higher incidence of atherosclerosis leads to a suspicion of coronary artery disease. In certain centers equipped with the technology, patients with an atypical clinical picture can undergo initial screening with multidetector CT or CRMI. In either circumstance, CAA would be a finding (Figure 6).
Figure 6. Diagnostic protocol proposed in patients over 35 years of age with suspected coronary artery anomaly. CRM indicates cardiac magnetic resonance imaging; CT, computed tomography; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
HOW IS THE INITIAL COURSE OF A CORONARY ANOMALY IDENTIFIED?
This is one of the most complex points of the study of CAA and it constitutes a fundamental aspect in risk identification.
To begin with, we have to take into account the fact that the name and nature of a coronary artery are defined by the territory they supply, not by their origin. Thus, a coronary that arises from right sinus of Valsalva and branches off to supply the territories of the anterior descending and circumflex (Cx) arteries, it is not a right coronary artery but a left main coronary artery with origin in the wrong sinus. When a coronary artery arises from the wrong sinus, the name, nature and even the function remain the same; only its origin and initial course are anomalous.2
After emerging from the wrong sinus of Valsalva, an anomalous coronary artery can reach its normal supply territory via at least five different routes2,52:
1. Retrocardiac, behind the mitral and tricuspid valves.
2. Retroaortic, usually followed by the Cx with origin in right sinus of Valsalva or in right coronary artery, adjacent to posterior aortic wall, in the groove between the atrium and aorta (transverse sinus) (Figure 3).
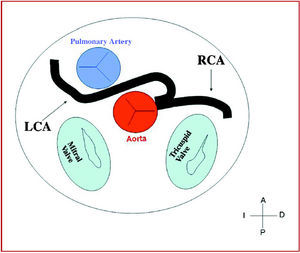
3. Preaortic or interarterial (between the aorta and the pulmonary artery), in which the anomalous right coronary, left anterior descending or left main coronary artery cross the septum or the aortopulmonary space. This is the course that has most frequently been related to signs of ischemia and/or sudden death (Figures 7 and 8).
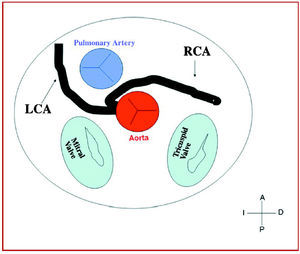
Figure 7. Diagram showing the interarterial course of a right coronary artery (RCA) originating in controlateral sinus.
Figure 8. Diagram showing the interarterial course of a left coronary artery (LCA) originating in controlateral sinus.
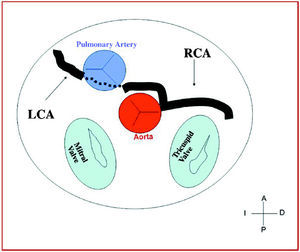
4. Intraseptal, through the upper portion of the intraventricular septum. It is intramyocardial in the majority of cases and is recognized on angiography because of the systolic narrowing, similar to a myocardial bridge, and because there usually are 1 or 2 septal perforator branches in this zone (Figure 9).
Figure 9. Diagram showing the intraseptal course of a left coronary artery (CI) originating in right sinus of Valsalva.
5. Precardiac or prepulmonary, characterized by its subepicardial location, in the anterior wall of the right ventricle outflow tract or infundibulum. This passage is usually followed by an anomalous right coronary, left main stem or left anterior descending coronary artery.
Other possible courses or even several courses in a given patient have been reported.2
How can these initial courses be identified by coronary angiography? Initially, it was considered that the introduction of a guide wire into the pulmonary artery followed by coronary arteriography in lateral projection would be sufficient to determine the relationship between the anomaly and the large vessels. However, this technique is of limited value since, for example, in a lateral view, both the septal and the interarterial courses would appear to be located posterior to the pulmonary artery and anterior to the aorta when, in reality, the septal course is located caudally to both vessels.53
Different coronary angiographic features for the recognition of the anomaly and its initial course, even prior to visualization, have also been reported. In this regard, Page et al54 propose two signs that enable us to recognize an anomalous origin of the Cx and its initial course:
1. The "unperfused myocardium" sign. During the selective opacification of left coronary artery, we can observe an avascular area in the posterolateral zone of left ventricle that indicates the anomalous origin of the Cx. It is first necessary to inject a contrast medium into left coronary sinus to rule out the involvement of ostia independent of the anterior descending and Cx arteries. However, if the identification of the Cx is not clear, we should remember that it is more common to observe the Cx originating in right coronary sinus or in right coronary artery than the presence of separate ostia in left coronary sinus.55
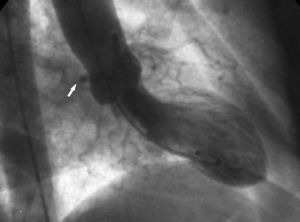
2. The "aortic root" sign. In ventriculography, the right anterior oblique projection shows the contour of the anomalous Cx and follows its course as it passes behind right coronary sinus (Figure 10).
Figure 10. Ventriculography in right anterior oblique projection showing the contour of the anomalous circumflex artery (arrow) passing behind right coronary sinus; this is known as the aortic root sign or Page's sign.
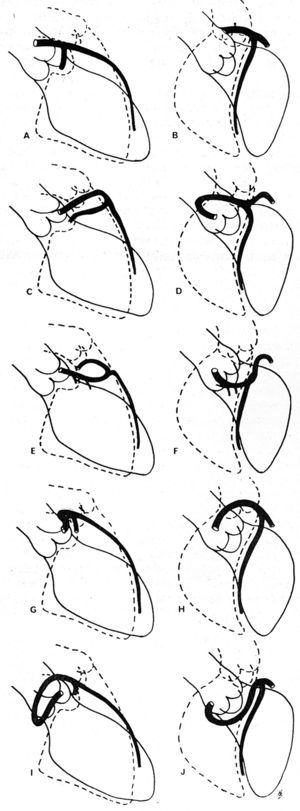
In 1985, Ishikawa et al,56 followed by Serota et al in 1990,57 proposed a series of coronary arteriographic criteria intended to define the relationship, along the initial course, between the anomalous vessel and the aorta and the pulmonary artery (Figure 11). These authors attempted to identify the different courses on the basis of the orientation of the "loop" formed by the anomalous coronary artery with the rest of the coronary tree56 or by applying the concept of the "dot" (visualization of the contrast column of the CAA which, because of the turn it makes, adopts the morphology of a dot57).
Figure 11. Illustrations of coronary angiographyc images of the left anterior descending and proximal circumflex coronary arteries in right anterior oblique projection (A, C, E, G, I) and in left anterior oblique projection (B, D, F, H, J), showing the different possible initial courses of a left coronary artery originating in right sinus of Valsalva. A and B represent a normal coronary artery; C and D show anterocranial "loop," described in the anterior course; E and F: anterocaudal "loop," septal course; G and H: posterocraneal "loop," interarterial course; I and J: posterocaudal "loop," retroaortic course. Taken from Ishikawa et al.56
In many cases, the identification of the initial course of the CAA by means of coronary angiography is difficult since this technique provides a two-dimensional view of the coronary tree, which is a complex three-dimensional structure. Interventional cardiologist experienced in the diagnosis of CAA, the coronary angiography may be sufficient for the correct identification of the course. However, given their low incidence, most interventional cardiologists have a limited experience in the study of CAA. Thus, in the vast majority of cases, they are not properly described. In a recent registry involving 13 Spanish hospitals, the initial course of the CAA reported was not identified in the majority of cases.16
Thus, on many occasions, the identification of the initial course of a CAA with origin in the wrong sinus requires an additional imaging technique. As we mentioned above, both TTE and TEE can be employed for this purpose but, given the increasing availability in our hospitals of noninvasive techniques that provide a more correct and exact definition of the coronary anatomy and its relationship to adjacent structures in patients who may be candidates for surgery, we opt for CMRI or multidetector CT.35,37,47,49
WHAT IS THE BEST THERAPEUTIC APPROACH?
This is one of the most controversial aspects of this condition since no official guidelines have been established in this respect.
Once the CAA has been diagnosed and characterized, it would appear to be logical to attempt to determine whether or not it is provoking myocardial ischemia. If the patient has had a myocardial infarction (with no demonstrable relation to atherosclerosis) or has undergone resuscitation for sudden death attributable to the anomaly and is under 35 years of age, most authors recommend revascularization.24,26,35
A thallium stress test or echocardiography under drug or exercise-induced stress demonstrating reversible ischemia in the territory of the anomaly will help in the decision-making process.36
What should be done in the case of young, asymptomatic individuals in whom CAA is discovered incidentally if risk criteria are met, but stress testing produces no evidence of ischemia? First, the patient should adopt a sedentary lifestyle.26,35
Corrado et al compared sudden death in young athletes and nonathletes and only those attributed to CAA or to arrhythmogenic right ventricular dysplasia were associated with exertion.58 With respect to treatment, revascularization is controversial, but there are authors who opt for this approach since, in these patients, sudden death is unpredictable and may be the first sign of CAA.24,26,34,35 Some authors support yearly follow-up visits in these cases, with echocardiograms or thallium stress tests, and base their decisions on the results.7,36 After surgical treatment, the patients enjoy a normal lifestyle, including participation in sports.25,34,49
In patients over 35 years of age, the decisions must be made on an individual basis. The risk of sudden death is lower but, since CAA can provoke symptomatic myocardial ischemia, in some cases revascularization is performed (Figure 12).24,26
Figure 12. Therapeutic protocol proposed for coronary anomalies with origin in contralateral sinus of Valsalva in the presence of risk factors.
Some groups are investigating the utilization of certain diagnostic protocols (intracoronary echocardiography to assess the anomalous coronary ostium at rest and after dobutamine infusion or fluid overload)59 or the utilization of intracoronary pressure wires to detect ischemia.60
As can be observed, until official guidelines are established in this matter, the most reasonable approach would appear to be, first, to confirm whether the age of the patient and the anatomy of the CAA are associated with risk and, second, to verify that the coronary ischemia (presented by the patient or demonstrated) is clearly provoked by the anomaly.
HOW SHOULD REVASCULARIZATION BE PERFORMED?
Logically, it is necessary to deal with each case on an individual basis according to coronary anatomy; thus, revascularization can be either surgical or percutaneous.
Surgical revascularization involves aortocoronary bypass, ostial reimplantation or the unroofing technique, which frees the intramural segment of the CAA by means of an incision in the wall shared by the CAA and the aorta, thus creating a new, larger orifice in the appropriate sinus.61,62 There is a great deal of controversy concerning surgical revascularization in these patients, especially in young people. Aortocoronary bypass grafting has been strongly criticized7,63 because of its "limited" patency and the inevitable competitive flow between the bypass and the CAA. Thus, the unroofing technique, which would appear to be more physiological,62 is increasingly being adopted.
Percutaneous intervention, with implantation of stents in the region of the compression between the large vessels and in the anomalous ostium, has been performed in several cases, in adults, with short-term success.64 To date, surgical revascularization is preferred in young patients and the percutaneous procedure is performed only in adults.26,62-64 Evidently, in both cases, long-term follow-up is necessary.
IS MEDICAL TREATMENT AN OPTION?
The lack of series and controlled studies makes it difficult to respond to this question. There are reports involving the 2-year and 5-year follow-up in patients who or patients who refused surgery, in whom the approach was either expectant management or treatment with beta-blockers, and among whom there were no cases of sudden death.65-68 However, some authors argue against the decision to initiate "lifelong" treatment in children and adolescents.7
MAJOR UNSOLVED PROBLEMS
The true risk of sudden death associated with each anomaly is unknown.5 The reason for this is that the risk is calculated on the basis of autopsy studies, which do not indicate the real risk of a person dying as the result of a CAA, but suggest the possibility that a person who has experienced sudden death has a CAA.7 Moreover, the calculated risks may be overestimated with respect to the general population since they are obtained from series of competitive athletes who, as has been reported, have a two-fold higher risk of sudden death than a sedentary person.69 We know that the risk of sudden death due to a CAA is greater in young people; in fact, one therapeutic approach or another is recommended depending on whether the patient is less than 30 or 35 years of age. These cut-off points are based on the few studies dealing with this matter23,25 and, thus, should be considered merely indicative. For this reason, the therapeutic approach should always be decided on an individual basis.
If the surgical solution described above is adopted, it must be taken into account that there are no series in which the course of the patients was studied for more than two years. To the uncertainty concerning the patency of the bypass the possible damage to the aortic valve that the unroofing procedure can produce is added. In this respect, there are reports of aortic insufficiency or even valve replacement following this procedure.7,70 We must remember that cardiac surgery always entails the risk of neurological complications, the incidence of which in young people has been estimated to be around 2.3%.71
Finally, the guidelines for the selection of competitive athletes with cardiovascular anomalies recommend the exclusion of patients with CAA from all competitive sports.51 Once again, the data from a population of athletes have been extrapolated to the general population. In an adolescent, the term "competitive" may be difficult to define. Moreover, forbidding an adolescent or young adult to participate in gym classes or the sport he or she may enjoy can cause more damage than benefits. Perhaps strenuous sports should be avoided, but not a less demanding sport.7
CONCLUSION
Coronary arteries originating in the wrong sinus of Valsalva constitutes the group of CAA most commonly associated with myocardial ischemia and, in particular, with sudden death.
The index of suspicion of this anomaly should be high in young patients showing clinical signs of angina, dyspnea or exertional syncope. In these cases, TTE should be performed in the attempt to identify the ostia and the initial course of both coronary arteries.
We should clearly define the initi al course of every anomalous coronary artery, using imaging techniques such as TTE, TEE or, preferably, multidetector CT or CMRI, according to the characteristics of our hospital.
We must give priority to the establishment of national and international registries that enable us to determine the incidence of these anomalies, as well as the associated rates of morbidity and mortality, prognosis, treatment, possible genetic or environmental influences and, in short, learn as much about this as we can.
It is necessary to carry out a long-term follow-up of patients who have undergone surgery for CAA, especially young people. In any case, revascularization (surgical or percutaneous) may be a valid for patients with this type of disease.
ACKNOWLEDGEMENTS
We thank Dr Joaquín Alonso and Dr Gabriel C. Fernández for their aid in obtaining the multidetector computed tomography images.
Correspondence:
Dr. R. Barriales-Villa.
Servicio de Cardiología. Complexo Hospitalario de Pontevedra.
Mourente-Montecelo, s/n. 36071 Pontevedra. España.
E-mail: rbarrialesv@inicia.es