Current 2022 guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery (NCS) of the European Society of Cardiology (ESC) are an update on the previous guidelines published in 2014.1,2 These guidelines are intended to standardize, with an evidence-based approach, the perioperative cardiovascular management of patients undergoing NCS. This document is aimed at all the physicians (not only cardiologists) involved in this scenario. Indeed, the guidelines are endorsed by the European Society of Anaesthesiology and Intensive Care (ESAIC). Despite of the lack of randomized clinical trials in the perioperative setting, current guidelines increase the evidence of recommendations compared with the previous document, and are focused on simplifying the management of patients undergoing NCS with new algorithms and practical figures. This article, drafted by a group of experts at the suggestion of the Guidelines Committee of the Spanish Society of Cardiology (SEC), attempts to highlight the most relevant novelties, positive or controversial aspects and social implications of the new guidelines in order to improve local clinical practice.
COMMENTS ON METHODOLOGYThe structure of these guidelines is the same as other ESC guidelines published in the last few years. New and revised recommendations are highlighted at the beginning and, at the end, the messages of “What to do” and “What not to do” are summarized. Unfortunately, there are few clinical trials on perioperative risk assessment and generally the levels of recommendations are B and C. A key point is risk stratification, both surgery-related and patient-related, for which the guidelines are fairly concise and simple, based on consensus with the ESAIC. The document specifies when to perform an electrocardiogram (ECG), determine biomarkers, or perform an echocardiogram, stress test, or coronary anatomy study. The authors have summarized the general management of NCS in a simple figure, although there are multiple sections that address the perioperative risk evaluation in different specific cardiovascular diseases (CVDs). In relation to perioperative pharmacological management, there is more solid scientific evidence based on clinical trials, especially for beta-blockers and antithrombotic therapies. The recommendations for the perioperative management of certain pharmacological therapies, such as sodium-glucose co-transporter-2 (SGLT-2) inhibitors, diuretics, and angiotensin receptor-neprilysin inhibitors, are new and useful. Perioperative complications to be aware of and how to manage them are summarized. In general, it is a very complete guide, with aspects of great clinical usefulness and certain controversial aspects, which should be consulted to adapt to individual practice environments.
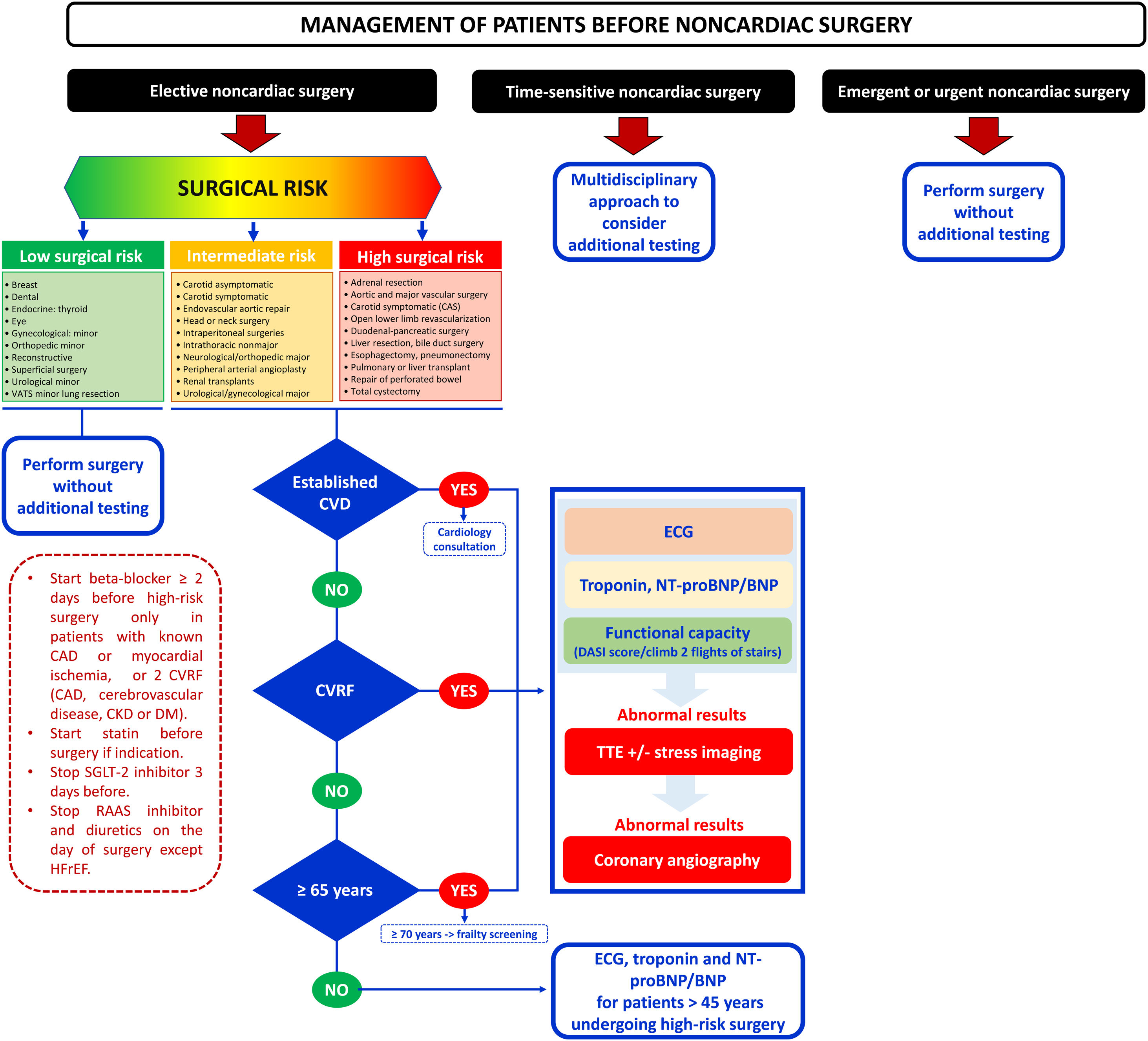
NOVELTIESClinical risk evaluationAs a novelty, compared with the 2014 guidelines, a new flowchart is proposed in which the timing of surgery (immediate, urgent, time-sensitive, or elective) is considered (figure 1). An individualized and multidisciplinary approach is recommended in time-sensitive NCS. In patients requiring elective NCS, performing a preoperative risk assessment is recommended, ideally at the same time as the intervention is proposed. Three risk groups are identified based on age (65 years), cardiovascular (CV) risk factors (including hypertension, smoking, dyslipidemia, diabetes, and family history of CVD), or established CVD. A heart check-up, including ECG, biomarkers (high-sensitivity-cardiac troponin [hs-cTn; Class I], brain natriuretic peptide [BNP]/N-terminal proBNP [NT-proBNP; Class IIa]) and assessment of functional capacity based on activities of daily living or scales (Class IIa) is recommended in even apparently healthy people older than 65 years before intermediate- or high-risk NCS. In patients aged 45 to 65 years without signs, symptoms, or history of CVD, ECG and biomarkers should be considered before high-risk NCS (IIaC). This novel strategy compared with previous guidelines aims to identify perioperative myocardial injury (PMInj), which is the most common CV complication associated with higher mortality within the first month after surgery. To quantify functional capacity, reference is made to a more objective way of estimating cardiac risk with the Duke Activity Status Index performed in the Measurement of Exercise Tolerance before Surgery study.3
Management of patients undergoing noncardiac surgery according to the ESC 2022 guidelines. CAD, coronary artery disease; CKD, chronic kidney disease; CVD, cardiovascular disease; CVRF, cardiovascular risk factors; DM, diabetes mellitus; ECG, electrocardiogram; HFrEF, heart failure with reduced ejection fraction; RAAS, renin-angiotensin-aldosterone system; SGLT-2, sodium-glucose co-transporter-2; TTE, transthoracic echocardiogram; VATS, video-assisted thoracoscopic surgery.
In terms of complementary tests, the indications for preoperative ECG are simplified, without recommending its routine performance in low-risk patients undergoing low-risk NCS. For biomarkers, the level of recommendation has been expanded to IB for the determination of hs-cTn in patients with known CVD, CV risk factors (including age ≥ 65 years), and symptoms suggestive of CVD before intermediate-high-risk NCS, and at 24hours and 48hours afterwards. The recommendation has also been upgraded to IIaB to measure BNP or NT-proBNP in the same situation.
In terms of transthoracic echocardiogram (TTE), routine assessment of left ventricular ejection fraction (LVEF) prior to surgery is still not recommended because that preoperative TTE before high-risk NCS did not reduce the risk of postoperative complications. In addition, reference is made to preoperative focused cardiac ultrasound (FOCUS) examination with a hand-held ultrasound device for the assessment of murmurs, hemodynamic instability, ventricular function, and dyspnea. This guideline makes it clear that stress testing alone should only be considered a valuable alternative for diagnosing obstructive coronary artery disease (CAD) if noninvasive imaging tests are not available, or for assessing functional capacity when the clinical history is ambiguous.
For invasive angiography, the indications are simplified, similar to the nonsurgical setting, and it may be considered in stable chronic coronary syndrome (CCS) patients undergoing elective surgical carotid endarterectomy. Evidence for the use of coronary computed tomography angiography for improved risk estimation is added but the document points out that the results may need to be supplemented by noninvasive functional testing to decide the need of revascularization.
There is a specific new section on the patient perspective, highlighting that time should be allowed to address concerns and provide evidence-based information on risk-benefit trade-offs and surgical treatment options, as well as to allow patients to engage in shared decision-making. In contrast to the previous 2014 guidelines, there is special reference to frailty in a specific section. The document suggests that perioperative assessment of elderly patients (> 70 years) requiring intermediate- or high-risk NCS should include frailty screening.
Pharmacological management in the perioperative settingLifestyle modifications and control of CV risk factors (including smoking cessation> 4 weeks before NCS) are encouraged. Regarding new recommendations on pharmacological risk reduction strategies, the guidelines consider temporary discontinuation of diuretics on the day of NCS as well as interrupting SGLT-2 inhibitors for at least 3 days before intermediate- and high-risk NCS, to minimize risk of euglycemic ketoacidosis.
Single antiplatelet therapy with aspirin for primary prevention is still recommended to be discontinued for NCS. Regarding secondary prevention, the new guidelines state that the only subgroup of patients that may benefit from aspirin continuation, in the absence of a very high bleeding risk, is those with previous percutaneous coronary intervention (PCI). If the bleeding risk outweighs the ischemic risk, aspirin should be interrupted at least 3 days before NCS (even 7 days before NCS in high bleeding risk procedures such as neurosurgery).
The recommended duration of dual antiplatelet therapy (DAPT) has changed in clinical practice guidelines published in recent years.4–6 Accordingly, new perioperative recommendations regarding DAPT no longer distinguish between bare metal stents (BMS) and drug-eluting stents (DES). Observational evidence yields discordant results about the impact of P2Y12 interruption on ischemic events in patients undergoing NCS, and therefore no specific recommendations can be established. If possible, it is recommended to delay elective NCS until 6 months after elective PCI and 12 months after an acute coronary syndrome (ACS) (and minimum 1 month of DAPT after elective PCI and 3 months after high-risk ACS). In relation to the minimum duration of DAPT after an ACS, the authors of this review consider that in special situations the interruption of the P2Y12 inhibitor could be considered from the first month, especially with the evidence provided by the new DES. Once the P2Y12 inhibitor is discontinued, surgery should be performed with aspirin. The decision to adjust treatment prior to surgery should be made considering each patient and by consensus between the surgeon and cardiologist. Table 1 summarizes the recommended time interval for antiplatelet drugs before NCS.
Discontinuation of antiplatelet therapy before noncardiac surgery
| Antiplatelet drug | Interruption (days before surgery) |
|---|---|
| Aspirin | Patients with prior PCI: not interruption* |
| High bleeding risk surgery: 7 | |
| Clopidogrel | 5 |
| Prasugrel | 7 |
| Ticagrelor | 3-5 |
PCI: percutaneous coronary intervention.
The management of perioperative oral anticoagulation is one of the main novelties of the current document compared with that of 2014. Thus, the guidelines provide practical algorithms, and recommendations are in the same way as previous statements.7–9 Regarding vitamin K antagonists (VKA), restrictive use of bridging therapy is recommended (only in high thrombotic risk patients such as mechanical heart valve). Invasive procedures with low bleeding risk can be performed without VKA interruption, and the international normalized ratio should be monitored to maintain the lowest level in the therapeutic range. A practical approach of direct oral anticoagulant (DOAC) management in elective NCS is more detailed in these guidelines, including practical figures based on the drug used, periprocedural bleeding risk, and renal function. Regarding specific procedures, in interventions carrying a very high risk of bleeding, such as spinal or epidural anesthesia, or lumbar puncture requiring intact hemostasis, discontinuation of DOACs for up to 5 half-lives is recommended. The use of bridging therapy with heparin is not recommended (except in exceptional circumstances of high thrombotic risk). In general, DOACs can be restarted 6 to 8hours after interventions with rapid and complete hemostasis. When the risk of bleeding is higher, it can be postponed for up to 48 to 72hours, with evaluation of the use of heparin. There is no evidence to recommend the use of lower doses of DOACs in this context. These new guidelines include the use of specific agents for reversing DOAC in urgent NCS. When these drugs are not available, prothrombin complex concentrate should be considered, although there is no evidence on its safety and efficacy in this setting.
A subsection has been added on combined oral anticoagulant and antiplatelet therapy. In this situation, elective surgery should be postponed until antiplatelet therapy can be safely discontinued (6 months after elective PCI or 12 months after ACS), although it would be reasonable to individualize it with a multidisciplinary approach. In emergency surgery with high bleeding risk, operative measures to reduce bleeding and anticoagulation reversal strategies can be applied.
A new section of perioperative thromboprophylaxis has been included in the 2022 guidelines. When indicated, thromboprophylaxis should be initiated during hospital stay up to 12hours before surgery and continued postoperatively according to individual assessment of bleeding risk until the patient is fully mobilized or until hospital discharge. DOACs in the thromboprophylaxis dose could be an alternative to low molecular weight heparin after total knee and hip arthroplasty.
The concept of “patient blood management” (PBM) is a novelty included in current guidelines. The document provides recommendations on the “3 pillars” of PBM: preoperative hemoglobin and iron level optimization, intraoperative bleeding reduction, and transfusion optimization. In addition, perioperative goal-directed hemodynamic therapy is nowadays fully established, mainly to guide fluid therapy in patients undergoing high-risk NCS. Nonsteroidal anti-inflammatory drugs are not recommended in patients with CVD due to safety issues, even though postoperative pain is associated with myocardial injury in patients with CVD.
Specific diseasesCoronary artery diseaseThe guidelines quantify the degree of risk in patients with CAD according to their baseline risk profile (prior CAD, older age, and recent acute coronary event), the risk of the intervention to be performed and the emergency of the surgery. There is no scientific evidence to support the proactive search and, where appropriate, prophylactic revascularization of CAD in asymptomatic patients with CCS. The role of revascularization in symptomatic patients is controversial and will depend on 3 factors: symptom control with medical treatment, the ischemic area detected, and the involvement of the left main coronary artery. In the case of urgent NCS and the coexistence of an ACS, the decision on the intervention must be made individually by a Heart Team. It is recommended to revascularize at least the responsible artery, use DES, and postpone elective surgery for at least 3 months.
Heart failureThe new guidelines maintain the need for prior evaluation of ventricular function with TTE and natriuretic peptides (if they have not been recently evaluated), although with a IB recommendation compared with the IA of the 2014 version, as well as maintaining the need to optimize and maintain treatment according to the heart failure (HF) treatment guidelines with an IA level of evidence. The novelty that is presented in the text is the need to monitor blood volume during the intervention, even with invasive methods if necessary. New clinical scenarios are mentioned, such as patients with ventricular assist devices due to advanced HF; in these cases, the guidelines stress the need for a multidisciplinary team and surgery in a center with access to expert ventricular assist teams (IC).
Valvular heart diseaseIn patients with severe symptomatic aortic stenosis, the guidelines recommend aortic valve replacement or transcatheter aortic valve implantation (TAVI), reserving percutaneous valvuloplasty as the last therapeutic option for patients not eligible for TAVI (Class IIb). In patients with asymptomatic severe aortic stenosis, valve procedure prior to NCS is only indicated if LVEF is <50% in the setting of high-risk interventions. In symptomatic or asymptomatic patients with low LVEF and primary mitral regurgitation, surgical repair before NCS is recommended. However, in patients with secondary mitral regurgitation, optimal medical treatment of the underlying cause is suggested as the first step; if symptoms persist, surgical or percutaneous valve repair is indicated. Unlike the 2014 version, the current guidelines recommend surgical treatment in patients with severe aortic regurgitation, who require the valvular intervention before an intermediate- or high-risk NCS. However, no significant differences are established in the management of significant mitral stenosis, or the management of patients with prostheses or prophylaxis of infectious endocarditis.
Known or newly diagnosed arrhythmias, adult congenital heart diseases, pericardial diseaseCatheter ablation is recommended as the first option to treat supraventricular tachycardia prior to elective high-risk NCS (IIaB), and recurrent ventricular tachycardia under optimized medical treatment prior to elective NCS (IB). The new guidelines include additional data on perioperative management of cardiac implantable electronic devices in NCS, including the first reference to leadless pacemakers and subcutaneous implantable cardioverter-defibrillators (ICD). Elective mid- to high-risk NCS in patients with congenital heart disease is recommended to be performed in experienced centers (IC). Finally, the 2022 guidelines include recommendations on patients with pericardial disease (not mentioned in the 2014 edition).
There are no major novelties regarding the approach to patients with hypertension before NCS, except for the recommendation to avoid perioperative blood pressure fluctuations, which is upgraded to IA (IIa in the 2014 version). Regarding peripheral artery disease (PAD), the new guidelines expand on this section by detailing an aspect that they share with the 2014 version: routine referral for cardiac work-up, stress testing or coronary angiography prior to elective surgery for PAD is not recommended (IIIC). In patients with cerebrovascular disease, the guidelines continue to suggest studying and treating carotid stenosis only in patients with neurological symptoms 6 months prior to NCS. The assessment of obesity prior to NCS is new, as is evaluation of glycated hemoglobin (HbA1c) to rule out diabetes mellitus. Finally, in a new COVID-19 section, the guidelines emphasize that there is no evidence to carry out CV screening prior to surgery in patients after COVID infection, but they do recommend delaying elective surgery until symptoms and comorbidities have stabilized.
Perioperative cardiovascular complicationsPerioperative myocardial infarction/injuryThe guides differentiate 2 entities:
Perioperative myocardial injury. This is any elevation of hs-cTn in the first 48hours after NCS, without the need for symptoms, ECG changes or contractile alterations on imaging tests. Given the analgesia after this type of surgery, 90% of patients with perioperative myocardial injury do not have symptoms. This entity has been associated with mortality of up to 10% per month and there is no evidence that any intervention reduces its incidence. The document adds a subentity called myocardial injury following NCS (MINS), in which the most likely underlying cause is CAD. It remains to be determined whether there are more adequate hs-cTn thresholds than simple elevation above the threshold to discriminate the prognosis of these patients.
Perioperative myocardial infarction. Diagnosis of perioperative myocardial infarction requires hs-cTn elevation in the first 48hours with additional criteria (symptoms, ECG changes, imaging test, or coronary angiography).
Atrial fibrillation (AF) and arrhythmias. A new section specifically on postoperative AF (POAF) has been included in these guidelines, with a management protocol. Regarding the approach to postoperative stroke, the introduction of a recommendation with level of evidence IA on treatment with DOACs in patients who develop POAF is novel, since it is the most frequent cause of perioperative stroke. There is a lack of evidence on appropriate antithrombotic therapy in patients with postoperative venous thromboembolism because recent major surgery or trauma has been a classic contraindication in previous trials of thrombolytic or anticoagulant therapy. Anticoagulation, preferably with DOACs, should be initiated, depending on postoperative renal function and bleeding risk, as early as possible for at least 3 months. If the patient is unstable, thrombolysis or surgical or percutaneous mechanical thrombectomy should be considered if there is a high risk of bleeding. Finally, no differences in patients with spontaneous acute myocardial infarction, HF or tako-tsubo syndrome after NCS compared with usual care are suggested.
POSITIVE ASPECTSClinical risk evaluationThe indications for the performance of complementary tests prior to NCS have been simplified or clarified. The document mentions how the interpretation of BNP/NT-proBNP concentrations as quantitative markers of HF with evolutionary cutoff points may facilitate HF detection, optimal intraoperative monitoring and initiation or optimization of HF treatment after surgery. The document also stresses that excessive testing may cause an unnecessary and unpredictable delay in an already planned surgical intervention, adding an independent procedural risk to the overall risk. A very positive point is the special mention of frailty in this guideline.
Specific diseasesFor the first time, the guidelines directly address how to perform interventions in patients with CAD. If PCI is indicated before NCS, the use of new-generation DES is recommended over BMS or balloon angioplasty. A minimalistic approach with plain balloon angioplasty and delayed stenting may be considered only in the unlikely combination of undeferrable NCS and concomitant ST-segment elevation myocardial infarction with an indication of PCI. The positive aspects of these guidelines regarding patients with HF is the prioritization of monitoring and treatment of these patients before NCS, in the moments prior to surgery, during the surgery, and in the postoperative period. New clinical scenarios such as obstructive hypertrophic cardiomyopathy are included, emphasizing even more the need to maintain blood volume and avoid vasodilation. As positive aspects, these guidelines include percutaneous techniques for the treatment of significant aortic stenosis and mitral regurgitation to reduce surgical risk. Regarding PAD, the guidelines distinguish between 2 types of NCS whose risks differ notably, and therefore so do the recommendations for their management: vascular surgery and nonvascular surgery. The guidelines clearly state that urgent NCS should not be delayed due to any arrhythmia, excluding life-threatening arrhythmic events. An illustration is provided to guide unipolar electrocautery configuration (patch placement) in supra-umbilical surgeries to minimize electromagnetic interferences.
General risk-reduction strategiesThe guidelines clarify the use of beta-blockers, renin-angiotensin system (RAAS) inhibitors, and statins in patients before NCS. Perioperative continuation of beta-blockers or statins is recommended in patients currently receiving this medication. Perioperative continuation of RAAS inhibitors may be considered in patients with stable HF, and their withdrawal is recommended in the remaining situations to prevent hypotension. Like recent guidelines on CAD, the new guidelines reflect the possibility of shortening DAPT, if deemed safe in terms of the patient's ischemic risk, avoiding unnecessary surgical delays, and lowering bleeding risk.4–6The main positive aspect of these guidelines regarding oral anticoagulation is the downgrading of bridging therapy. The only exceptions are mechanical heart valves and very high thrombotic risk patients. Another important point raised by these guidelines is the use of DOAC reversal agents in this setting. This may strengthen the feeling of safety in the use of these drugs. The recommendation of a validated tool, such as the Caprini score, for venous thromboembolism risk assessment may improve accurate patient selection for this therapy. Regarding PBM, many perioperative recommendations are strong and easy to implement, such as hemoglobin measurement or anemia treatment in advance surgery, mostly with iron.
Perioperative cardiovascular complicationsA new specific management of POAF is provided: the concept of increased risk of clinical AF and stroke following an episode of POAF is introduced, and oral anticoagulation is recommended following POAF based on risk scales with reassessment at 3 months of follow-up (IIaB).
CONTROVERSIAL ASPECTSClinical risk evaluationThere is no reference to telemedicine, which could have a role in the preoperative approach of patients with risk factors or established CVD. No specific recommendation is made on the role of nurses in the different phases of the disease, especially in patients undergoing time-sensitive NCS, such as cancer patients. In terms of risk scores, the document indicates that there is significant variability in the predicted risk of cardiac complications using different risk-prediction tools, and it is therefore unclear which one should be used in preoperative assessment of patients or whether it would be better to use a combination of several. Despite the special mention of frailty in these guidelines, some factors are not mentioned that are important in determining patients’ preoperative risk, such as very low or very high body mass index values, anaemia and immune status, among others, which can influence patients’ underlying CVD and CV risk factors, favoring the development of cardiovascular complications after NCS.
Specific diseasesThe role of stress testing is not defined in any group of valve diseases before NCS in the current guidelines. Additionally, there is no mention of the management of patients with significant tricuspid regurgitation, which has been associated with higher postoperative morbidity and mortality. It would be useful to remember the definition of PAD (patients with an ankle-brachial ratio of <0.9, or those who have been previously revascularized) used in the 2014 guidelines but absent in the current document. The document mentions the possible usefulness of low doses of rivaroxaban (2.5mg/12h) + aspirin in patients with PAD undergoing vascular surgery to reduce postoperative thrombotic events based on the VOYAGER PAD trial.10 However, because of the increase in the rate of major bleeding in the rivaroxaban + aspirin arm, the net clinical benefit casts doubt on such a recommendation. In patients with MINS and low bleeding risk, the guidelines recommend treatment with dabigatran (110mg/12h) from 1 week after NCS, based on the results of MANAGE trial.11 Its use for this indication in our environment seems unlikely. Finally, ablation of AF and isthmus-dependent flutter is not mentioned as a potential rhythm control strategy prior to elective NCS, despite a IA/IIA indication in the 2020 AF guidelines.
General risk-reduction strategiesPreoperative initiation of beta-blockers remains a matter of concern. The current guidelines recommend starting this drug at least 1 week before surgery (starting with a low dose and with dose titration for a target heart rate of 60-70 bpm.) in patients with> 2 risk factors (CAD, cerebrovascular disease, renal insufficiency, or diabetes mellitus). The document acknowledges that there are no data about perioperative management of aspirin monotherapy in patients with TAVI, and therefore its withdrawal should be assessed according to the bleeding risk of the intervention. Moreover, the role of bridging therapy with antiplatelets is unclear: although their use is generally not recommended, the guidelines do not specify which patients benefit from this treatment. There is a lack of well-powered studies to evaluate the role of platelet function testing to guide the strategy for treatment in NCS patients on antiplatelet therapy. Regarding the use of recombinant human erythropoietin together with iron preoperatively, further studies are needed to allow a recommendation. Another controversial aspect of postoperative management is how to perform effective analgesia, since the safety of nonaspirin nonsteroidal anti-inflammatory drugs, is not well established.
Perioperative cardiovascular complicationsThe guidelines recommend systematic hs-cTn measurement before and after intermediate and high-risk NCS in patients≥65 years, with CV risk factors or CVD. Any elevation is a warning sign that should trigger an investigation. However, in clinical practice, this can be a problem. The terms perioperative myocardial injury/perioperative myocardial infarction (PMI) are artificial or controversial, because these guidelines are also intended to guide noncardiologist physicians. A classification of perioperative infarction of cardiac or extracardiac origin might be more useful. Prevention of POAF remains challenging and the lack evidence precludes strong recommendations.
IMPLICATIONS FOR CLINICAL PRACTICE IN SPAIN AND LOCAL SOCIOECONOMIC CONDITIONSClinical risk evaluationThe new recommendations should facilitate the assessment and flow of patients between anesthesia, surgery and cardiology services and lead to better functioning and reduction of cardiology consultations by limiting or clarifying the use of traditionally overused complementary tests such as ECG and TTE. The guidelines emphasize the need for a patient-centered approach that ensures therapeutic optimization and minimizes perioperative risk. However, the guidelines do not provide recommendations on how to implement this organizational need in clinical practice and do not include a proposal for interaction/coordination between specialists. Equally, they do not detail the criteria for referring patients to other professionals, nor do they specify the time for performance of complementary examinations. Although NT-proBNP and hs-cTn measurement seems to us a promising approach, it may lead to overuse of periprocedural measurements without solid current evidence and could unnecessarily delay planned surgeries and overload the cardiology services of our health care system, and therefore lead to an excessive economic cost.
Specific diseasesThe specific approach in the management of patients with previous CAD who will undergo elective NCS in these guidelines could allow the unification of action criteria in all cardiology services in Spain. Additionally, it could also allow the development of clinical care protocols in NCS processes involving other specialties to make them more efficient. Likewise, savings are expected in tests and interventions that have not shown benefits in this context. Patients with HF schedule to undergo NCS will often require cardiological care for presurgical evaluation with support during and after the intervention. This has an impact on the need for resources to be able to deliver this care. Risk discrimination between vascular vs nonvascular NCS and the management proposed will facilitate the implementation of more efficient protocols based on the surgical process, allowing noncardiologist specialists to establish the preferred evaluation pathways, and avoiding unnecessary consultations and procedures. Perioperative management in ICD carriers is simplified in the new guidelines. The use of a magnet is prioritized as the recommended method to inhibit ICD therapies during NCS (prior guidelines recommended deactivation by device programming). This change provides safer and more efficient perioperative management.
General risk-reduction strategiesBridging therapy is a widespread practice in our setting. Many physicians may be unaware of the evidence of the bleeding risk associated with this approach. This lack of knowledge, together with therapeutic inertia and fear of the appearance of thrombotic events in these patients, make it difficult to abandon this strategy. These guidelines may help to spread the message that we have enough evidence to safely discontinue oral anticoagulants when indicated without bridging. This will undoubtedly lead to a reduction in bleeding events and better perioperative management. The implementation of PBM programs implies the reorganization of the preoperative assessment, in order to diagnose anemia and/or iron deficiency and potential treatment.
Perioperative cardiovascular complicationsAs the guidelines point out, the determination of hs-cTns before and after noncardiac surgery will mean a high rate of PMI diagnosis. This recommendation would entail a modification in the standards of care in surgical and anesthetic services, likely involving a cardiologist consultation that cannot always be guaranteed.
CONCLUSIONSThe new guidelines on the cardiovascular assessment and management of patients undergoing NCS provide simple and practical key messages to facilitate daily clinical decisions. A new flowchart with general assessment of patients before NCS focused on ECG and biomarkers attempts to facilitate preoperative management. A practical approach of antithrombotic management in elective NCS is more detailed in these guidelines, including practical figures based on the drug used and periprocedural risk. The guidelines also highlight the perioperative management in the most frequent CVDs and focus on the specific care according to the risk of the patient's prior clinical condition. Finally, the document includes practical recommendations on the management of postoperative complications, and a new section on perioperative myocardial infarction/injury is highlighted.
FUNDINGNone.
CONFLICTS OF INTERESTThe conflicts of interest declaration documents of all authors can be seen in the supplementary data.
SEC Working Group on the 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery: David Vivas (coordinator), Sergio Raposeiras-Roubin (coordinator), Guillermo Aldama, Felipe Bisbal, Clara Bonanad, Raquel Ferrandis, Román Freixa-Pamias, Rosa Fernández-Olmo, Ainhoa Robles-Mezcua, José Francisco Rodríguez Palomares, Amalia Sillero-Sillero.
SEC Guidelines Committee: Rut Andrea, Pablo Avanzas, Gemma Berga, Araceli Boraita, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos Subirá, Juan José Gómez Doblas, Pilar Mazón, Domingo Pascual, Juan Sanchis, José M. de la Torre, David Vivas, José L. Ferreiro (President).
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.10.010
The names of all the authors of the article are listed in alphabetical order in Appendix A.
See related article: https://secardiologia.es/cientifico/guias-clinicas/miscelanea/13801-2022-esc-guidelines-on-cardiovascular-assessment-and-management-of-patients-undergoing-non-cardiac-surgery