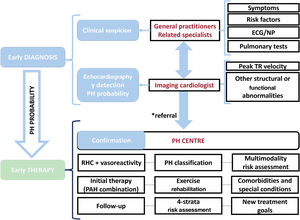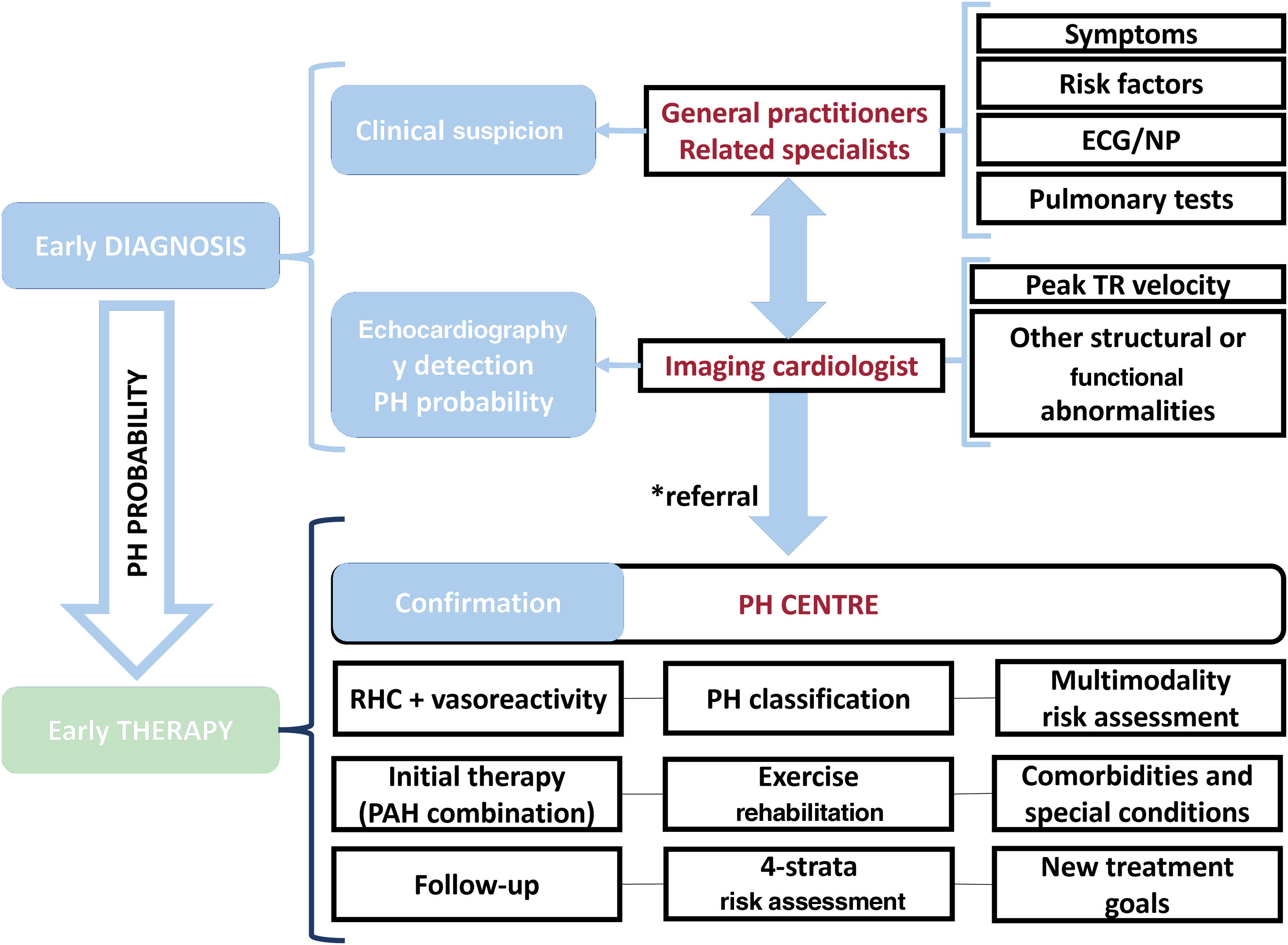
The 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines for the diagnosis and treatment of pulmonary hypertension (PH)1 include several novelties that are the focus of this comment. In addition, we would like to emphasize the need for improvement of both early diagnosis and early treatment, based on establishing an organized, collaborative, and multidimensional approach, from a local and regional perspective. This collaborative team approach directly involves first-line physicians, echocardiography, and specialized PH centers. This concept is illustrated in figure 1.
Collaborative team approach for the diagnosis and management of patients with PH. The asterisk (*) indicates intermediate/high probability of PH, in the presence of risk factors for PAH, or a history of pulmonary embolism. ECG, electrocardiogram; NP, natriuretic peptides; PH, pulmonary hypertension; RHC, right-sided catheterization; TR, tricuspid regurgitation.
The cutoff value of mean pulmonary arterial pressure (mPAP) that defines the diagnosis of PH has been reduced to> 20mmHg. This change is based on new data obtained with right heart catheterization (RHC) performed in a relatively limited number of healthy individuals with a wide age range. However, several large retrospective studies have confirmed the prognostic association of an mPAP> 20mmHg justifying the change in threshold. The implications of this new definition in terms of referral of patients to specialized PH centers and use of diagnostic and therapeutic resources should be evaluated in Spain. In addition, the guidelines underscore the importance of including pulmonary vascular resistances (PVR) and pulmonary arterial wedge pressure (PAWP) in the definition of precapillary PH. Two Wood units is the upper limit of normal PVR and the lowest prognostically relevant threshold of PVR, while 15mmHg is the cutoff value of PAWP. Based on the combinations of the cutoff values of mPAP, PVR and PAWP, several hemodynamic definitions of PH are established (table 1). It is important to underscore the need to perform the RHC following standardized and precise methodology.
Hemodynamic definitions of pulmonary hypertension
| Definition | Hemodynamic characteristics |
|---|---|
| Pulmonary hypertension | mPAP> 20 mmHg |
| Precapillary pulmonary hypertension | mPAP> 20 mmHgPAWP ≤ 15 mmHgPVR> 2 WU |
| Isolated postcapillary pulmonary hypertension | mPAP> 20 mmHgPAWP> 15 mmHgPVR ≤ 2 WU |
| Combined post- and precapillary pulmonary hypertension | mPAP> 20 mmHgPAWP> 15 mmHgPVR> 2 WU |
| Exercise pulmonary hypertension | mPAP/cardiac output slope between rest and exercise> 3 mmHg/L/min |
mPAP, mean pulmonary arterial pressure; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; WU, Wood units.
The main changes in the classification of PH include the addition of the subgroups “nonresponders at vasoreactivity testing” and “acute responders at vasoreactivity testing” to the group of idiopathic pulmonary arterial hypertension (PAH). However, some patients with heritable PAH (HPAH) or drug- or toxin-associated PAH (DPAH) may also be included in the subgroup of “acute responders at vasoreactivity testing”. Furthermore, the DPAH subgroup has been simplified and the use of methamphetamines and dasatinib has been definitively associated with PAH. The group “PAH with features of venous/capillary (pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis [PVOD/PCH]) involvement” has been included in group 1 (PAH). PAH and PVOD/PCH share a broadly similar hemodynamic profile, clinical presentation and similar causes and associated conditions, although some are more frequently associated with more pronounced venous/capillary involvement. This feature is associated with poorer prognosis, and worse response to PAH therapy, with risk of pulmonary edema. In Spain—mainly among the Romani ethnic group—2 there is a high incidence of hereditary PVOD due to biallelic mutations in the eukaryotic translation initiation factor 2α kinase 4 (EIF2AK4) gene; thus, it demands specific awareness in diagnosis. Finally, the name of PH group 3 has been changed from “sleep-disordered breathing” to “hypoventilation syndromes” since nocturnal obstructive sleep apnea itself is generally not a cause of PH, but PH is frequent in patients with hypoventilation syndromes, which can also behave with daytime hypercapnia.
EARLY DETECTION PULMONARY HYPERTENSION ALGORITHM AND EARLY REFERRALA new diagnostic algorithm has been designed aiming at earlier detection of PH. Expedited referral is recommended for high-risk patients at any level of the diagnostic process. It is mandatory to identify underlying diseases, especially left heart and/or lung disease as well as comorbidities, to ensure proper classification and guide treatment. The proposed diagnostic algorithm should be considered in patients with unexplained dyspnea or signs/symptoms suggesting PH and includes 3 steps:
Step 1. Suspicion. Initial evaluation (usually performed by primary care physicians) should include a comprehensive medical and familial history, thorough physical examination (including blood pressure, heart rate, and pulse oximetry), BNP/NT-proBNP, and resting electrocardiogram. This first step may raise suspicion of a cardiac or respiratory disorder causing symptoms different from PH.
Step 2: Detection. Includes noninvasive lung and cardiac testing. Echocardiography is very important as it assigns a level of probability of PH and allows identification of other cardiac disorders. If causes other than PH are identified, patients should be managed accordingly.
Step 3. Confirmation. Patients should be referred to a PH center for further evaluation when an intermediate/high probability of PH is detected and in the presence of risk factors for PAH, or a history of pulmonary embolism. Establishing the differential diagnoses and distinguishing between the various causes of PH according to the current clinical classification is mandatory. The PH center is responsible for performing an invasive assessment (RHC).
The presence of any of the following warning signs is associated with worse outcomes and requires immediate intervention: rapidly evolving or severe symptoms (World Health Organization Functional Class [WHO-FC] III/IV), clinical signs of right ventricular failure, syncope, signs of low cardiac output, poorly tolerated arrhythmias, and hemodynamic instability. Such cases must be managed as inpatients and referred to a PH center immediately.
The guidelines also emphasize the importance of fluid collaboration between first-line, specialized medicine, and PH centers to allow earlier diagnosis, and therefore early treatment, which may improve outcomes. A proposed approach to facilitate an earlier diagnosis includes:
- •
Screening asymptomatic, high-risk groups: populations with a high prevalence of PH such as patients with scleroderma, BMPR2 mutation carriers, first-degree relatives of patients with HPAH and patients undergoing assessment for liver transplant or transjugular portosystemic shunt. Screening programs should adopt a multimodal approach to increase PH detection. There is general agreement in performing screening annually in at-risk populations.
- •
Early detection of symptomatic patients in at-risk groups with conditions such as portal hypertension, human immunodeficiency virus infection, and nonsystemic sclerosis connective tissue disease, where prevalence rates do not support asymptomatic screening.
- •
Population-based strategies by deploying early detection approaches in pulmonary embolism follow-up and breathlessness clinics.
Tools to increase PH screening include echocardiography, NT-proBNP, electrocardiography, pulmonary functional tests (lung diffusion capacity for carbon monoxide [DLCO] and forced vital capacity [FVC]/DLCO ratio) and cardiopulmonary exercise testing (CPET). Echocardiography is recommended with a class IB to assign an echocardiographic probability of PH, based on an abnormal tricuspid regurgitation peak velocity (TRV) and the presence of other signs suggestive of PH. The current threshold for TRV (> 2.8 m/s) remains recommended (class IC) for assessing the echocardiographic probability of PH with the updated hemodynamic definition. Depending on the probability of PH on echocardiography, further testing should be considered, such as CPET. CPET may identify characteristic features of exercise limitation due to pulmonary venous disease or suggest an alternative diagnosis.
The most relevant updates in recommendations on early diagnosis of PH in at-risk populations are:
- •
In asymptomatic patients with scleroderma and a history of the disease of> 3 years, an FVC ≥ 40%, and a DLCO <60%, the DETECT algorithm is recommended to identify patients with PAH (class IB).3 In symptomatic patients, exercise echocardiography, CPET or cardiac magnetic resonance may be considered to aid decisions to perform RHC (class IIbC).
- •
In patients with persistent or new-onset dyspnea or exercise limitation following pulmonary embolism, further diagnostic evaluation to assess for CTEPH/chronic thromboembolic pulmonary disease is recommended (class IC). The optimal timing for assessing symptoms to aid early detection of CTEPH may be 3 to 6 months after acute pulmonary embolism, coinciding with routine evaluation of anticoagulant treatment. For symptomatic patients with mismatched perfusion lung defects beyond 3 months of anticoagulation for acute pulmonary embolism, referral to a PH/CTEPH center is recommended (class IC).
- •
Echocardiography is recommended in patients with liver disease or portal hypertension with signs or symptoms suggestive of PH, and as a screening tool in patients evaluated for liver transplant or transjugular portosystemic shunt (class IC).
The role of multimodality imaging in the risk stratification of patients with PH has been emphasized in the new guidelines. Together with right atrial area and the presence of pericardial effusion, right ventricular (RV)-pulmonary arterial (PA) coupling estimated by the echocardiographic tricuspid annulus plane systolic excursion (TAPSE)/systolic pulmonary arterial pressure (sPAP) ratio appears to be a valuable novel parameter for risk assessment in patients with PAH. This measure has recently demonstrated a good correlation with RV-PA coupling assessed by invasive pressure-volume loops and to predict outcome. The cutoff values incorporated in the guidelines to differentiate low, intermediate, and high risk are> 0.32, 0.19 to 0.32 and <0.19mm/mmHg, respectively, which have demonstrated independent prognostic value.1 In addition, 3 new parameters obtained by cardiac magnetic resonance imaging have been incorporated into the risk stratification: RV ejection fraction, stroke volume index (SVI), and RV end-systolic volume index (RVESVI). Cardiac magnetic resonance imaging is considered the gold-standard for the evaluation of RV structure and function, which constitute the main prognostic factors in PAH. Moreover, RV ejection fraction may deteriorate despite an improvement in hemodynamics and several studies have demonstrated that changes in cardiac magnetic resonance imaging measurements provide similar or even more prognostic information than changes in hemodynamic parameters. Therefore, it was completely expected that these 3 variables would have been incorporated. The thresholds for RV ejection fraction (> 54%, 37-54%, <37%) stem from a recent study showing that these cutoffs identified low, intermediate, and high risk of 1-year mortality.4 The rationale for setting the SVI thresholds (> 40, 26-40, <26mL/m2) is less justified; at this point the guidelines refer to a previous study in which a cutoff of 38mL/m2 better discriminated prognosis5 and another study that reported a worse survival for patients with a SVI ≤ 25mL/m2.6 Finally, the incorporation of RVESVI is based on several studies demonstrating its independent prognostic value, and the cutoff of 54mL/m2 to identify high risk is based on a study finding that this threshold was associated with a higher risk of mortality.4
RECOMMENDATIONS ON INITIAL DRUG THERAPIES–SIMPLIFICATION–AND FOLLOW-UP STRATAConcerning the treatment of patients with PH group I, including idiopathic, heritable, associated with drugs and toxins, or associated with connective tissue disease, for which there is more evidence, we have a completely new treatment algorithm for PAH that has been simplified, with a clear focus on risk assessment, cardiopulmonary comorbidities, and treatment goals. In this algorithm, initial combination therapy and treatment escalation at follow-up when appropriate are current standards. In addition to targeted drug treatment, the comprehensive management of patients with PAH includes general measures and supportive therapy (e.g., anticoagulation, diuretics), which have not been significantly changed. However, the role of exercise deserves to be highlighted, as the recommendation of supervised exercise training in stable PAH patients with the best standard of pharmacological treatment has been upgraded (class IA) based on additional evidence showing the beneficial impact of exercise training on exercise capacity, quality of life, WHO-FC, and peak oxygen uptake (VO2) compared with the standard of care.
The initial treatment decision should be based on disease type and severity and also in the presence of cardiopulmonary comorbidities such as conditions associated with an increased risk of left ventricular diastolic dysfunction and signs of parenchymal lung disease, often associated with a low DLCO. Patients with PAH who respond favorably to acute vasoreactivity testing may respond to calcium channel blockers. After a positive vasoreactivity test and while on calcium channel blockers, the safety and efficacy of the treatment should be evaluated and the reactivity test and RHC should be repeated after 3 to 6 months of therapy. Satisfactory chronic reactivity response is defined by a World Heart Organization functional class I-II and with an mPAP <30mmHg and PVR <3 WU. In patients without cardiopulmonary comorbidities, a reclassification looking at the risk of the patients by using a 3 strata risk score is needed to decide initial treatment. In low- or intermediate-risk patients, initial combination therapy with a phosphodiesterase 5 inhibitors (PDE5i) and an endothelin receptor antagonist (ERA) is recommended, with tadalafil combined with ambrisentan or tadalafil with macitentan having the highest recommendation class (IB). In high-risk patients, the addition of parenteral prostacyclin analogs should be considered.
It must be considered that the efficacy of PAH drugs has only been demonstrated in patients with mPAP ≥ 25mmHg and PVR> 3 WU and no recommendations are available for the revised hemodynamic definition (mPAP> 20mmHg, PVR> 2 WU) or for patients with exercise PH. Pending further data, the role of PAH drugs in these patients needs to be explored. Patients at high risk of developing PAH, namely systemic sclerosis or family members of patients with HPAH, should be referred to a PH center for individual decision-making.
RISK ASSESSMENT AND TREATMENT GOALS IN FOLLOW-UPThe implementation of a 4-strata risk assessment tool (4S) for follow-up is one of the most relevant novelties of the present guidelines. It will change clinical practice and the timing of drug indication. This new model defines 2 different intermediate risk levels, low and high, allowing a more precise discrimination within the intermediate risk category, which comprises up to 70% of all patients.7 In addition, recent data from national registries8–10 show that the mortality risk is higher than previously thought, specifically for the intermediate- and intermediate-high risk groups. Accordingly, 1-year mortality risks have been updated for each stratum as follows: low risk: 0% to 3%, intermediate-low risk: 2% to 7%, intermediate-high risk: 9% to 19%, and high risk:> 20%.
The same recent studies from national registries agree that WHO-FC, 6-minute walking distance, and BNP/NT-proBNP, which are all noninvasive variables, are the strongest prognostic predictors.8–10 This new evidence, in association with the 4S, will allow an easy but precise risk assessment of patients during follow-up. Additional imaging and hemodynamic variables should be used for risk stratification. Also, at any stage, other nonmodifiable factors such as age, sex, disease type, comorbidities, and kidney disease should be considered.
During follow-up, a risk-based, goal-oriented treatment approach is still recommended in the present guidelines. Even though it will be challenging in most cases, achieving low risk should be the goal for every patient as it warrants the best long-term prognosis.10 The new guidelines establish the following recommendations for follow-up treatment decisions:
- •
For patients at intermediate-low risk despite receiving ERA/PDE5i therapy, consider adding selexipag as first choice. Also, switching from PDE5i to riociguat might be considered to optimize intervention on nitric oxide pathway.
- •
For patients at intermediate-high or high risk while receiving oral therapies, adding iv epoprostenol or iv/sc treprostinil must be considered as first choice. At the same time, the patient should be referred for lung transplant evaluation. Only in patients considered not suitable for parenteral prostanoids in an individual assessment (older patients, sum of comorbidities, treatment rejection), consider adding selexipag as first choice or switching from PDE5i to riociguat to optimize oral therapy.
A very appropriate decision of the guidelines’ task force has been to highlight the treatment of patients with cardiopulmonary comorbidities. These are found predominantly in elderly patients and include risk factors such as obesity, diabetes, coronary artery disease, systemic hypertension (left heart phenotype) or a history of chronic smoking and low DLCO (cardiopulmonary phenotype). These patients respond worse to PAH medication, are less likely to reach low-risk status, have a higher mortality risk and are more likely to discontinue this medication due to efficacy failure or low tolerance. Accordingly, in patients with PAH presenting at intermediate or high risk of death, the decision to add PAH medication to the indicated initial monotherapy with a PDE5i or an ERA, should be individualized based on limited evidence.
Regarding the recommendations for general measures, this edition emphasizes the beneficial impact of exercise training, correction of iron status in the presence of iron-deficiency anemia and immunization of patients against SARS-CoV-2 in addition to influenza and Streptococcus pneumoniae.
Specific recommendations for the group of patients with PAH after corrected adult congenital heart disease are introduced for the first time, with an initial treatment strategy and follow-up similar to that recommended in patients with idiopathic PAH. In Eisenmenger syndrome, bosentan is the recommended treatment in symptomatic patients, unlike the 2020 ESC guidelines for the management of congenital heart disease in adults, in which the indication was based on a 6-minute walking distance of <450 m and treatment with ERA (nonspecific) was recommended. In the absence of response to initial ERA, combination therapy is recommended. Finally, there are no long-term therapy recommendations for patients with PH and elevated PVR that contraindicate shunt closure.
RECOMMENDATIONS ON LEFT-SIDED HEART DISEASE-RELATED PULMONARY HYPERTENSIONPH and RV dysfunction are commonly present among patients with left heart disease and are associated with a poor prognosis. Left heart disease is probably the leading cause of PH, being responsible for approximately 70% of cases. Left heart disease includes patients with heart failure (reduced, mildly reduced, or preserved left ventricular ejection fraction), left-sided valvular disease and congenital or acquired cardiovascular conditions leading to postcapillary PH.
In addition to the modifications on hemodynamic definitions of PH, diastolic pressure gradient is no longer used to distinguish between isolated postcapillary PH and combined post- and precapillary PH because of conflicting data on the prognosis of patients with left heart disease.
Diagnostic key points in evaluating suspected PH in left heart disease include: a) diagnosis and follow-up of the underlying left heart disease; b) evaluation for PH and patient phenotyping; and c) invasive hemodynamic evaluation when indicated. Current guidelines strongly recommend an accurate diagnosis followed by treatment optimization of left heart disease before considering invasive assessment of PH. Furthermore, patients with left heart disease and suspected PH should be evaluated following the diagnostic strategy for PH. The guidelines provide a practical clinical tool to phenotype patients and help diagnose and decide which patients should undergo a full PH work-up.
While invasive assessment for PH is usually not indicated in patients with a strong likelihood of left heart disease as the leading cause of PH or with established underlying left heart disease and mild PH, these new guidelines introduce 2 new indications for RHC: a) in patients with severe tricuspid regurgitation, with or without left heart disease, before surgical or interventional valve repair; and b) suspected combined post- and precapillary PH with a severe precapillary component, where further information will aid phenotyping and treatment decisions. Likewise, additional testing during RHC may help to uncover patients with heart failure with preserved left ventricular ejection fraction (HFpEF) with normal resting PAWP but an abnormal response to exercise or fluid challenge.
The effects of new medical therapies for heart failure (angiotensin receptor-neprilysin inhibitor or sodium-glucose cotransporter-2 inhibitor) on PH, through reverse remodeling of the left ventricle, need further investigation.
Finally, although drugs approved for PAH are not recommended in patients with PH-left heart disease, the guidelines do not provide any recommendation for or against using PDE5i in patients with HFpEF and combined post- and precapillary PH. In contrast, there is clear recommendation against the use of PDE5i in patients with HFpEF who have isolated postcapillary PH.
CHRONIC THROMBOEMBOLIC PULMONARY DISEASE–NEW CONCEPTThe term chronic thromboembolic pulmonary disease (CTEPD), PH group 4, is introduced as a different clinical entity and includes patients with or without PH at rest who present dyspnea on exertion, mismatched perfusion defects on ventilation/perfusion scintigraphy and chronic, organized, fibrotic clots persisting after 3 months of anticoagulation on computed tomography pulmonary angiography or digital subtraction angiography.11 Patients with PH due to CTEPD are referred to as patients with CTEPH. Patients without PH at rest could report symptoms due to exercise PH and/or increased dead space ventilation. To diagnose CTEPD, induction of PH during exercise needs to be demonstrated.
In the diagnostic algorithm for patients with suspected CTEPD, ventilation/perfusion scintigraphy is the most effective tool to rule out the disease while computed tomography pulmonary angiography, digital subtraction angiography and selective segmental angiography, cone-beam computed tomography and area detector computed tomography are used for interventional planning and guidance.
The treatment algorithm for CTEPH has been modified, including multimodal therapy (surgery, PH medication, balloon pulmonary angioplasty [BPA]), which can be applied either simultaneously or sequentially. Lifelong therapeutic doses of anticoagulation are recommended in all patients with CTEPH and vitamin K antagonists are specifically recommended in patients with underlying antiphospholipid syndrome. While riociguat is recommended in symptomatic patients with inoperable CTEPH or persistent/recurrent PH after pulmonary endarterectomy (PEA) (class IB), off-label use of drugs approved for PAH may be considered in symptomatic patients with inoperable CTEPH (class IIb). Selected symptomatic patients with CTEPD without PH can be treated with PEA or BPA, with clinical and hemodynamic improvement both at rest and exercise. BPA has received a class IB recommendation in patients who are inoperable or have residual PH after PEA. These interventions should be performed in CTEPH centers defined by a high volume of interventions:> 50 PEA/y and> 30 patients/y undergoing BPA or> 100 BPA/y. These interventional volumes have been associated with improved outcomes although they may not be widely achievable in many European countries, including Spain. Accordingly, the guidelines acknowledge the need to adapt the definitions to each country highlighting the need for accredited centers with highest interventional volumes and good outcomes. The Spanish Society of Cardiology (SEC) and the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) propose the accreditation of PH centers with 3 levels of complexity: basic, specialized, and high complexity.12
Finally, the clinical benefits of pulmonary artery denervation are still under investigation, a fact that the guidelines mention as a gap in evidence.
PATIENT REPRESENTATIVESPH is a chronic disease in which patient associations play an important role. Patient associations provide educational and emotional support and can positively affect the self-confidence of patients as well as coping processes and attitudes. In this regard, the guidelines actively recommend that PH centers collaborate with patient associations on initiatives for patient empowerment and improve the patient and caregiver experience, attending to issues such as health learning, healthy lifestyles, well-being, and autonomy. As a novelty, patient representatives were actively involved for the first time in developing the current PH guidelines through the European Reference Networks (ERN) for rare diseases (ERN-LUNG), providing critical and constructive feedback, and ensuring that the patient voice is represented and considered as part of their decision-making.
POPULATION, INTERVENTION, COMPARATOR AND OUTCOME QUESTIONS–PRACTICAL QUESTIONS FOR PRACTITIONERSAn important novelty of these guidelines is the emphasis on 4 highly practical questions with direct consequences for clinical practitioners regarding each PH classification subgroup (population, intervention, comparator, and outcome [PICO] questions). The first refers to the initial treatment strategy for group 1 PH. Although the authors of the guidelines recognize that the quality of evidence is low, initial dual combination therapy with ERA and PDE5i is recommended rather than monotherapy for symptomatic patients with PAH. This recommendation is based on the results of the AMBITION trial,13 which demonstrated significantly lower risk of clinical-failure events (particularly hospital admissions) with the combination of ambrisentan and tadalafil than with any of them in monotherapy.
The second PICO question refers to the use of PDE5i in patients with combined post- and precapillary PH associated with HFpEF. However, in this case, no recommendation could be made for or against the use of PDE5i. This is due to the absence of randomized clinical trials specifically addressing this issue. Two randomized clinical trials enrolled patients with PH associated with HFpEF with conflicting results. One trial including patients with a predominantly isolated postcapillary PH profile reported negative results, as sildenafil had no effect on hemodynamics.14 The other trial, including patients with a predominantly combined post- and precapillary PH profile, reported positive results, as sildenafil improved hemodynamics, RV function, and quality of life.15 Accordingly, the guidelines highlight this gap in knowledge where new clinical trials including such patients may lead to new evidence that will impact future recommendations. In contrast, the guidelines clearly state the recommendation against the use of PDE5i for patients with HFpEF and isolated postcapillary PH profile.
The third PICO question addresses the use of oral PDE5i in patients with PH associated with interstitial lung disease (group 3 PH), which received a class III recommendation in the 2015 guidelines. There is an important knowledge gap in this regard. Current evidence arises from case series and registries, preventing the task force from drawing any definitive conclusions (resulting in a IIB recommendation). The document stresses the need to refer patients to a center of expertise in PH in order to provide a patient-tailored therapeutic approach. Importantly, the use of PH drugs in patients with nonsevere group 3 PH is not recommended.
Regarding PICO question 4, the document pays attention to the role of PH drugs prior to BPA in patients with CTPEH not suitable for PEA (group 4 PH), for which no recommendations were provided in the 2015 guidelines. The topic is even more relevant due to the new class IB recommendation for BPA in inoperable patients with CTEPH, which may presumably increase the number of procedures performed. Current evidence is this regard is scarce and includes barely a single randomized clinical trial and 2 single-center observational studies. In these studies, PH drugs prior to BPA showed a moderate improvement on pulmonary hemodynamics with a favorable safety profile. These results have translated to a “conditional recommendation” for the use of PH drugs prior to BPA in the new guidelines.
CONCLUSIONSThe key points of the 2022 ESC guidelines for the diagnosis and management of PH1 can be summarized as follows:
- •
PH is now defined by a mean PA pressure> 20mm Hg at rest. The new definition of PAH also implies a pulmonary vascular resistance> 2 Wood units and PAWP ≤ 15mm Hg.
- •
A simplified diagnostic algorithm for PH has been proposed to support early detection and early referral/treatment, following a 3-step approach, and based on a collaborative network:
- -
suspicion by first-line physicians
- -
detection by echocardiography
- -
confirmation in PH centers with RHC
- •
The initial treatment algorithm for PAH includes comprehensive risk assessment, cardiopulmonary comorbidities, and treatment goals.
- -
Multimodality imaging risk assessment (echocardiography and magnetic resonance imaging) is included in the 3-strata risk-stratification assessment.
- -
Initial combination therapy and treatment escalation at follow-up when appropriate are current standards.
- -
A new 4-strata risk assessment tool (4S) is used for goal-oriented treatment decisions during follow-up.
- -
Supervised exercise training in stable PAH patients with the best standard of pharmacological treatment is recommended (class IA).
- -
Cardiopulmonary comorbidities must be addressed simultaneously.
- •
PH group 2 needs treatment optimization of left heart disease before assessment of PH can be considered. Drugs approved for PAH are not recommended and there is no consensus for the use of PDE5i in patients with HFpEF and combined post- and precapillary PH.
- •
In PH group 3 “sleep-disordered breathing” has been changed to “hypoventilation syndromes”. It is recommended to optimize the treatment of the underlying lung disease, with enrollment into pulmonary rehabilitation programs; PDE5 inhibitors may be considered when severe PH is associated with interstitial lung disease.
- •
The term chronic thromboembolic pulmonary disease (CTEPD), PH group 4, is introduced as a different clinical entity. The diagnosis of CTEPH must be improved, based on early suspicion at the time of an acute pulmonary embolism and during the systematic follow-up of these patients.
The treatment algorithm includes multimodal therapy with surgery, PH drugs, and BPA.
FUNDINGNone.
CONFLICTS OF INTERESTThe conflict-of-interest declaration documents of all authors can be seen in the supplementary data.
SEC Working Group for the 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: Victoria Delgado (coordinator), Domingo Pascual-Figal (coordinator), Esther Calero, Pilar Escribano, Ana García-Alvarez, María Lázaro, Manuel López-Meseguer, José M. Montero-Cabezas, Patricia Palau, Alejandro Recio-Mayoral, Joaquín Rueda, María Teresa Velázquez.
SEC Guidelines Committee: Rut Andrea, Pablo Avanzas, Gemma Berga, Araceli Boraita, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos Subirá, Juan José Gómez Doblas, Pilar Mazón, Domingo Pascual, Juan Sanchis, José M. de la Torre, David Vivas, José L. Ferreiro (President).
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.11.001
See related article: https://secardiologia.es/cientifico/guias-clinicas/insuficiencia-cardiaca-y-miocardiopatia/13912-2022-esc-ers-guidelines-for-the-diagnosis-and-treatment-of-pulmonary-hypertension
The names of all the authors of the article are listed in alphabetical order in Appendix A.
Corresponding author.
Email addresses: videlga@gmail.com (V. Delgado); dpascual@um.es (D. Pascual).