European Society of Cardiology (ESC) Guidelines are endorsed by the Spanish Society of Cardiology (SEC, Sociedad Española de Cardiología) and have been translated into Spanish for publication in Revista Española de Cardiología. In line with a policy implemented in 2011, every updated version of the Guidelines is published with an accompanying article that comments on the aims and methods recommended by the SEC Guidelines Committee.1
In the present article, an updated version of ESC Guidelines on the Management of Stable Coronary Artery Disease (CAD) is discussed.2 The Guidelines Committee created a working group with members proposed by the Sections on Ischemic Heart Disease, Hemodynamics, Clinical Cardiology, and Imaging, and a representative of the Spanish Society on Cardiac Surgery.
A word-for-word translation of the original English title of the Guidelines into Spanish would be “enfermedad arterial coronaria estable”. The Editorial Board of Revista Española de Cardiología has decided to use “cardiopatía isquémica estable” (stable ischemic heart disease) because this is the most customary and common term for the disease used by Spanish-speaking cardiologists. Apart from this semantic issue regarding the translation, the most important conceptual change in the updated Guidelines is that the term “stable angina pectoris” used in the former title has been replaced with this much broader term intended to include both symptomatic and asymptomatic patients with a previous or present history of confirmed or suspected stable CAD.
The first consideration raised by the present version of the Guidelines is caution. Despite recent advances in cardiology, only 21 (16%) of the 130 recommendations are based on a high level of evidence (A), whereas 65 (50%) are based on expert consensus (level of evidence C). Such findings confirm the need for further cardiology research focused on the disease.
Needless to say, this is an extremely important disease. According to recent data from the OFRECE study3 by the SEC Research Agency, a history of acute coronary syndrome (ACS) is found in 4.9% of individuals older than 40 years in Spain and a confirmed diagnosis of stable angina according to the Rose questionnaire can be established in 2.6%. Although such figures are substantially lower than the mean value in Europe, they clearly show that there are more than 1 100 000 individuals with ACS and about 300 000 individuals with angina in Spain.
Relevant and novel aspects of the Guidelines are summarized in Table 1, and questionable, nonspecified, and nondiscussed aspects are shown in Table 2.
2013 ESC Guidelines on the Management of Stable Coronary Artery Disease. Relevant and/or Novel Aspects
| General | • A new name for the Guidelines that can be applied to a wider and more realistic range of patients |
| • Significant gaps in scientific evidence. Only 16% of recommendations are type A and 50% are type C | |
| Diagnosis and assessment | • “A careful history remains the cornerstone of the diagnosis of chest pain” |
| • Renal function assessment by means of creatinine clearance (I B) | |
| • Annual monitoring of lipid and glucose metabolism and creatinine (I C) | |
| • Baseline echocardiography in all patients (I B) | |
| • Ischemia provocation tests not recommended for diagnosis in patients with PTP < 15% or > 85% | |
| • Imaging tests to detect ischemia recommended for patients with PTP 65-85%, ejection fraction > 50% with no typical angina or baseline ECG changes (I B) | |
| • Conventional exercise tests with ECG recommended for patients with a baseline ST-segment depression and patients receiving digoxin (III C) | |
| • Exercise tests are preferred to pharmacological stress tests (I C) | |
| • The first time CCT is considered to be a useful test, especially to rule out coronary artery disease in patients with low or intermediate risk | |
| • CCT is not recommended in patients with a previous revascularization or as a screening test for asymptomatic individuals with no suspected coronary artery disease (III C) | |
| • Risk stratification is differently defined and modified, so that it is solely based on mortality | |
| • FFR estimation as needed during coronary angiography is emphasized | |
| • “Microvascular angina” replaces the term “syndrome X” | |
| • Microvascular angina diagnosis should be based on objective changes | |
| Lifestyle and pharmacological management | • Use of phosphodiesterase 5 inhibitors is safe, except when concomitant therapy with any nitrate is used |
| • To prevent events: ASA and statins always; ACE-I (or ARB-II) only in patients with heart failure, high blood pressure, or diabetes mellitus (I A) | |
| • Risk factor control should be emphasized and maintenance antianginal therapy used in patients with microvascular angina (I B) | |
| Revascularization | • Emphasis on the need for consensus decisions on revascularization by a multidisciplinary team, the so-called Heart Team (I C) |
| • Clopidogrel pretreatment should not be used in patients with suspected CAD undergoing diagnostic catheterization (III A) | |
| • Very limited use of ticagrelor and prasugrel plus ASA in patients with CAD undergoing PCI (IIb C) | |
| • Routine use of platelet functional tests is not recommended in PCI | |
| • Use of second-generation drug-eluting stents is recommended (I A) | |
| • Duration of dual antiplatelet therapy in patients undergoing PCI: | |
| - 1 month after metal stents implantation (I A) | |
| - When drug-eluting stents are used, 6-12 months treatment is recommended (I B), although the possibility of a shorter duration (1-3 months) is mentioned for patients with a high bleeding risk, those requiring a surgical procedure, and those receiving anticoagulants (IIb C) | |
| • Levels of recommendation (I-III) or evidence (A-C) are no longer used when selecting PCI versus surgery for revascularization | |
| • Chronic occlusion therapy indications are scarcely mentioned and rather restrictive | |
| Special groups | • Diagnostic tests for microvascular or vasospastic angina should be used in women with angina whose coronary arteries show no angiographic lesions |
| • Screening exams for ischemia should not be used in asymptomatic patients with diabetes mellitus or chronic kidney disease |
ASA: acetylsalicylic acid; ARB-II: angiotensin II receptor blockers; CAD: stable coronary artery disease; PCI: percutaneous coronary intervention; ACE-I: angiotensin-converting enzyme inhibitor; PTP: pre-test probability; FFR: fractional flow reserve; CCT: coronary computerized tomography.
Aspects not including a class of recommendations or level of evidence are mentioned in the original Guidelines but no such classifying details are reported.
2013 ESC Guidelines on the Management of Stable Coronary Artery Disease. Questionable, Nonspecified and Nondiscussed Aspects
| Diagnosis and assessment | Straight invasive coronary angiography in patients with PTP > 85% and severe symptoms |
| A prognostic score system based on clinical history alone could not be developed | |
| Role of Holter monitoring in silent ischemia screening | |
| Very cautious approach when using CCT details for risk stratification | |
| Carotid intima-media thickness measurement (including atherosclerosis plaques screening), ankle-brachial index estimation, or coronary calcium measurement by CCT in asymptomatic patients with intermediate SCORE risk (IIa B) | |
| Lifestyle and pharmacological management | General warning on the risks of nonsteroidal anti-inflammatory drugs, but no recommendations for selecting specific drugs are reported |
| No discrimination among beta-blockers is mentioned, apart from the need for caution when using nevibolol and bisoprolol in patients with renal dysfunction | |
| Anti-anginal drugs: both calcium channel antagonists and beta-blockers are included as a first line anti-anginal therapy (I A), even though studies supporting the recommendation are very old and probably not applicable | |
| Long-acting nitrates, ivabradine, nicorandil and ranolazine are considered, but specific anti-anginal indications for each drug are not reported (IIa B) | |
| Specific indications are not reported, especially for ivabradine compared with beta-blockers and calcium channel antagonists with a negative chronotropic effect | |
| Revascularization | Definition of asymptomatic patients receiving optimal medical therapy is not reported, ie, which level of symptoms and which level of therapy |
| How to create a Heart Team, make decisions, and involve patients in decision-making is not mentioned | |
| Recommendations on revascularization for main artery disease are based on subanalyses, using SYNTAX score, which means that using such results to set definite guidelines is at least questionable | |
| Chronic occlusion therapy is scarcely mentioned and, in any case, only patients with a severe angina or large ischemic areas are considered | |
| Audits: the Guidelines make no mention of external audits on the results of surgical and interventional groups in different centers and publication of the results by health authorities is not discussed | |
| Extracorporeal versus off-pump surgery: the team's experience and risk level in patients undergoing surgery in a specific center should be considered when selecting the procedure |
PTP: pre-test probability; CCT: coronary computerized tomography.
Aspects not including a class of recommendations or level of evidence are mentioned in the original Guidelines but no such classifying details are reported.
We would highlight the reaffirmation in the present Guidelines that, “A careful history remains the cornerstone of the diagnosis of chest pain.”
Basic ExaminationsType I B indication for Doppler echocardiography in all patients can be controversial, and a repeated exam during the follow-up period is not recommended in the absence of clinical changes. Strictly speaking, it is true that in the absence of heart failure, arrhythmias, previous myocardial infarction, ECG changes, or murmurs, echocardiography is rarely informative. However, from our point of view, a comprehensive knowledge of the heart's baseline structure and function can be advisable in a patient with a serious disease that may result in complications and require long-term follow-up. In our opinion, the main barrier to routine use of echocardiography in all patients is the resulting increased health care burden, rather than strictly medical reasons.
We think that carotid artery ultrasound examination (IIa C), a technique advocated by other specialists rather than by cardiologists, is not so clearly warranted.
InvestigationsOne of the most significant and appropriate additions in the present Guidelines is a novel approach to the use of diagnostic investigations by means of a 3-step decision making algorithm, as follows:
- 1.
Estimation of pre-test probability (PTP) of CAD in a patient presenting with chest pain is the first step. The PTP can be easily calculated using Table 13 in the Guidelines, although final values can vary depending on the differing prevalences of CAD across European countries.
- 2.
Noninvasive tests aimed at establishing a diagnosis of CAD or nonobstructive atherosclerosis are the next step. The Guidelines clearly advise against performing diagnostic testing to detect ischemia in patients with a PTP < 15% (CAD is assumed not to be present) or > 85% (presence of CAD is assumed). From our point of view, this is a very reasonable approach, as cogently expressed in the following statement: “Testing may do harm if the number of false test results is higher than the number of correct test results.” All clinicians should apply such an approach. After all, we are simply admitting that absolute certainty regarding a diagnosis cannot be achieved and that a diagnosis is just a hypothesis about the nature of the disease in an individual patient.
- 3.
The third step, once a diagnosis has been reached, is to initiate optimal medical management and stratify the risk of further events, usually by means of noninvasive investigations. At this stage, the Guidelines recommend a straight coronar y angiography in 3 subgroups of patients: a) ventricular dysfunction and typical angina, b) PTP > 85% and conditions suggesting a high risk (annual mortality > 3%), and c) PTP > 85% and severe symptoms. We have no objection to the first 2 points. However, in our opinion, a straight coronary angiography solely based on “severe symptoms”, before offering medical treatment as an option for control, seems arguable because symptoms expression varies between individual patients and individual response to medical management is unpredictable. In patients with a 15% to 85% PTP, noninvasive approaches to detect ischemia should b e use d. Ideally, imaging stress tests are recommended as a first technique. Taking into account that a universally routine use of such tests is not possible, it should be considered indispensable in patients with a 66% to 85% PTP, nontypical angina, ejection fraction < 50%, and baseline ECG changes. Conventional exercise tests with ECG are restricted to patients with a 15% to 65% PTP, and are not recommended (III C) in patients with a baseline ST-segment depression or in patients receiving digoxin.
In our opinion, occasional use of conventional exercise tests with ECG is appropriate to assess ischemia symptoms and control, once medical treatment has been initiated. However, according to the Guidelines, its usefulness has not been formally assessed. It should be remembered that regular use of routine imaging stress tests in asymptomatic patients undergoing percutaneous or surgical revascularization has been reported to increase the number of further revascularizations; however, no improvement in prognosis was observed.4
Regarding noninvasive exams to evaluate coronary anatomy, coronary computerized tomography (CCT) has a high negative predictive value that allows the disease to be ruled out, although an excess of patients with an Agaston index > 400 can be found. No type I recommendation is given for CCT. However, CCT can be considered an alternative technique to imaging stress tests in patients with inconclusive findings or contraindications, and may rule out coronary disease in patients with a low PTP (15% to 50%) (recommendation II A).
In our context, it should be noted that use of CCT is restricted by its limited availability.
Risk StratificationIn this section, several clear concepts are presented in the Guidelines:
- 1.
Risk stratification allows the identification of patients with a high risk for e v ents who could po t entiall y b enef it fr om revascularization.
- 2.
Risk assessment includes only mortality.
- 3.
Risk definitions have been modified in the updated Guidelines. High risk is now defined as an annual mortality > 3% (in such patients, indication for revascularization should always be considered), moderate risk as 1% to 3% annual mortality, and low risk as an annual mortality < 1%.
A sequential strategy has been developed, based on clinical evaluation, baseline ventricular function assessment and stress testing, and finally an assessment of coronary anatomy only when such details are needed.
Although the importance of prognostic information provided by clinical details (previous history, risk factors, angina type, laboratory findings, and ECG) is highlighted, the authors admit that a scoring system cannot be developed based on the currently available evidence. Thus, such potentially valuable details are relegated to a modulating role in decisions based on data acquired in later steps. This remains a major gap in our knowledge.
As is classically the case, the main factors to be considered in risk stratification are ventricular function and extent of ischemia or coronary disease. Ventricular function assessment (usually by echocardiography) is confirmed as the best predictor for mortality, and a high-risk threshold is established at an ejection fraction ≤ 50%. The Guidelines and Annex include a number of tables that help to stratify risk in individual patients based on findings from ischemia detection tests. As a general rule, myocardial ischemia > 10% results in a high risk and a recommendation for coronary angiography evaluation.
The Guidelines are very cautious regarding use of CCT findings in risk stratification. Although added details on plaque morphology (“low” attenuation plaques) are very promising, their current usefulness for prognostic purposes is still uncertain. Thus, when considering CCT findings, an additional test for ischemia is wisely recommended before a patient is transferred to invasive coronary angiography (IIa C).
An important section not included in previous Guidelines focuses on asymptomatic patients with no known CAD. Seven recommendations (none of them being type I) for this subgroup of asymptomatic individuals with at least moderate risk according to SCORE are shown in Table 21 of the Guidelines. In patients with intermediate SCORE risk, carotid intima-media thickness measurement (including atherosclerosis plaque evaluation), ankle-brachial index estimation, or coronary calcium measurement by CCT are recommended (IIa B). Apart from other considerations (methodological, financial, and others) that could be discussed regarding such tests and other investigations in a screening setting, they only afford a new categorization of cardiovascular risk, which is solely based on applying primary prevention measures corresponding to the new risk threshold. An ischemia detection test, let alone invasive coronary angiography, is still inappropriate, because there is no evidence suggesting that such an aggressive approach could result in any prognostic improvement.
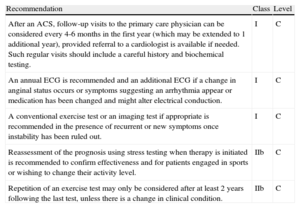
Asymptomatic patients with a known CAD are highly prevalent and show a higher risk for events. According to OFRECE study findings,3 in Spain there could be more than 800000 patients fulfilling such criteria. Thus, this group of patients results in a significant use of both cardiology and primary care resources, especially because no studies have been carried out to compare different follow-up strategies. Follow-up recommendations are summarized in Table 3, which has been adapted from Table 22 in the Guidelines. As shown, all evidences are classified as a level of evidence C, and a low grade of recommendation is ascribed to repeating an ischemia provocation test of any kind in the absence of clinical changes or breakthrough acute events (IIb).
Recommendations for Follow-up in Patients With Stable Coronary Artery Disease
| Recommendation | Class | Level |
| After an ACS, follow-up visits to the primary care physician can be considered every 4-6 months in the first year (which may be extended to 1 additional year), provided referral to a cardiologist is available if needed. Such regular visits should include a careful history and biochemical testing. | I | C |
| An annual ECG is recommended and an additional ECG if a change in anginal status occurs or symptoms suggesting an arrhythmia appear or medication has been changed and might alter electrical conduction. | I | C |
| A conventional exercise test or an imaging test if appropriate is recommended in the presence of recurrent or new symptoms once instability has been ruled out. | I | C |
| Reassessment of the prognosis using stress testing when therapy is initiated is recommended to confirm effectiveness and for patients engaged in sports or wishing to change their activity level. | IIb | C |
| Repetition of an exercise test may only be considered after at least 2 years following the last test, unless there is a change in clinical condition. | IIb | C |
The recommendation that follow-up exams after an ACS should be performed by primary care physicians seems controversial to us. In our opinion, a cardiologist should perform some kind of initial monitoring. Continued care should follow protocols based on consensus with the primary care physician, and hospital discharge reports should include some follow-up plan based on the local protocols, risk assessment, and patient characteristics. A recently reported SEC project, “Outpatient Management in Cardiology,” recommends cardiologist involvement, along with a primary care physician, over the initial 6-month to 1-year period, depending on individual ventricular function.
Angina With “Normal” Coronary ArteriesAs in the 2006 Guidelines, this disease entity is divided into 2 types: microvascular angina (replacing the term “syndrome X”) and vasospastic angina. The recommendations for diagnosis and therapy are difficult to assess; most of them are Class II recommendations and all are based on a level of evidence C, due to their confusing, changing, and inadequately studied clinical symptoms.
In patients not showing obstructive lesions in epicardial coronary arteries, the requirement that a microvascular angina diagnosis include a typical angina or a stress-induced atypical angina along with objective evidence of exercise-induced ischemia or a reduced coronary reserve in Doppler exam of anterior descending artery seems appropriate. The recommendation for vasospastic angina diagnosis is either an ECG when pain is present or Holter monitoring. If a coronary angiography is performed and no coronary lesions are observed, spasm-provocation tests with intracoronary acetylcholine or ergonovine are recommended (IIa C); this test is probably underused in most laboratories in our context. However, intravenous ergonovine tests are contraindicated when coronary tree anatomy is not known and in patients with significant lesions.
LIFESTYLE AND PHARMACOLOGICAL MANAGEMENTLifestyle and prevention recommendations in the updated Guidelines are based on recommendations in other recent guidelines on the management of dyslipidemia and on cardiovascular disease prevention. A specific recommendation is to follow a Mediterranean diet rich in olive oil or nuts, as recently demonstrated in a large Spanish study5. Surprisingly, specific aims for diabetes mellitus, cholesterol, and blood pressure, but not for heart rate, are included in the Guidelines on CAD.
Reminders regarding the safe use of phosphodiesterase-5 inhibitors for erectile dysfunction are of interest; the only absolute contraindication is a concomitant therapy with any nitrate. There are also important recommendations about avoiding nonsteroid anti-inflammatory drugs (NSAIDs) if at all possible, or using them at the lowest effective dose and only for the shortest possible period of time. There is no mention of the expert consensus6 recommending naproxen as the least harmful of the NSAIDs.
Regarding medical treatment, as in the previous version, 2 categories are considered: angina relief and improvement of prognosis. A new table (Table 27 in the Guidelines) reports adverse effects, contraindications, and interactions of anti-ischemic drugs.
Beta-blockers, previously considered to provide both symptoms relief and prognosis improvement, are now classified only as anti-anginal drugs in the present table. However, the text emphasizes that extrapolation of study data on the benefits to prognosis in postinfarction or heart failure patients would suggest that beta-blockers should be first-line anti-anginal drugs in CAD. We would emphasize that we agree on this concept: beta-blockers do not improve prognosis in these patients unless ventricular dysfunction is present. In spite of the various characteristics of individual beta-blockers (alpha selectivity, beta selectivity, intrinsic sympathomimetic activity, and others), they are used in clinical practice as if a general class effect were present. Some comments or recommendations on this issue would have been appropriate. We agree with the appropriate reminder of the partial renal elimination of nevibolol and bisoprolol, which means that carvedilol and metoprolol can be safer in patients with renal failure.
Unlike the previous version and the ACCF/AHA 2012 Guidelines, the present updated Guidelines include both calcium channel blockers and beta-blockers as a first-line recommendation with the same level of evidence (I A) as an anti-anginal therapy. The recommendation for calcium channel blockers shown in the table is surprising and does not match with the scant evidence available about these drugs, as appropriately described in the text.
Long-acting nitrates continue to be a second-line therapy (IIa B) because no evidence supporting their efficacy and safety has been reported, and a potentially harmful effect on endothelial function and oxidative stress has been suggested. Mixed into the same section of the table that includes long-acting nitrates, we also find ivabradine, nicorandil, and ranolazine; the choice between such drugs should be based on parameters such as heart rate, blood pressure, and tolerability. Each one is briefly described in the text, but indications based on patient characteristics are not clearly stated. At least some more detailed comments on the role of ivabradine in patients not able to tolerate beta-blockers or its use in combination with beta-blockers in patients with a high heart rate would have been appropriate. Moreover, the Guidelines seem to assume that calcium channel blockers with a negative chronotropic effect are always preferable to ivabradine, which appears to be arguable from our point of view, at least for certain subgroups of patients, such as those not having high blood pressure.
Regarding microvascular angina therapy, it is worth emphasizing that prognosis is not as good as traditionally thought in patients with proved ischemia and a correct diagnosis; this is because of a high stroke incidence, which could be due to the combination of a number of risk factors. Risk factor control and continued use of drugs that sometimes are withdrawn when such patients are classified as “functional” play a crucial role in disease management.
Chest pain associated with normal coronary arteries and no objective evidence of ischemia is a very common condition that is difficult to manage. It has not been clearly established who should be in charge of such patients, many of whom receive no definitive diagnosis —let alone medical solutions— despite multiple efforts (including repeated catheterizations). The Guidelines mention only in passing that clinical symptoms often correspond to extracardiac, organic, or functional diseases. In more than half the women undergoing coronary angiography because of symptoms consistent with angina, the coronary arteries are normal or have no significant lesions. Thus, the Guidelines state that some women not showing significant lesions have microvascular angina or vasospastic angina; this is the reason why spasm-provoking tests with ergonovine or acetylcholine and coronary vasodilation reserve studies are so important. Creating specific multidisciplinary units to help understand and manage such conditions could possibly be a useful proposal.
REVASCULARIZATIONThe revascularization section has a special structure. Instead of starting with indications for revascularization, the section begins with a description and discussions of the available techniques: percutaneous coronary intervention (PCI) and surgery. When indications (revascularization or medical treatment) are finally approached, the statement is the following: “The decision to revascularize a patient should be based on the presence of significant obstructive coronary artery stenosis, the amount of related ischemia, and the expected benefit to prognosis and/or symptoms.” Thus, symptoms relief, which is the main expecte d b enef it from revascularization techniques in most patients with CAD, is left in the last place and almost unnoticed. Furthermore, it is not even a starting point in the decision-making algorithm on revascularization shown in Figure 5 in the Guidelines. Some paragraphs later, the following is stated: “When technically feasible, with an acceptable level of risk and a good life expectancy, revascularization is indicated in chronic angina refractory to optimal medical therapy.” In practical terms, the problem lies in deciding the extent of symptoms that should be considered unacceptable to maintain a conservative approach and exactly what constitutes optimal medical therapy (in Table 28, optimal medical therapy is said to include the use of at least one drug for ischemia or angina relief, apart from secondary prevention drugs). Due to such an imprecise definition, large differences remain in revascularization rates across various teams, particularly regarding subjective interpretation of such aspects by teams that are more or less prone to interventionism. Therefore, the Guidelines report a number of detailed considerations on revascularization in post-myocardial infarction patients with left ventricular dysfunction, multivessel disease, a large ischemic territory, and main artery disease. Whereas the last 3 indications appear clear and well supported in the table of recommendations, with assigned grades being between I A and I B, the post-infarction element is rather confused (this may not be the most appropriate guideline to discuss this point).
Revascularization in Low Risk PatientsIn patients with stable angina and a documented ischemia who are classified into the “low risk” category after a careful clinical and angiographic evaluation, an initial strategy based on medical management is safe and should be the first choice. Such an approach should be especially considered for patients with severe comorbidities or a complicated anatomy. In our opinion, this is an appropriate consideration when taking into account that an initial candidate for medical management may well become a revascularization candidate when symptoms worsen; this happened, for example, in patients allocated to the medical management arm in the COURAGE study,7 with no negative impact on prognosis being observed. On the other hand, starting medical management with no angiographic confirmation in patients with a positive test for ischemia is now considered an appropriate strategy, at least while waiting for the results of the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) study, which are expected to be available in 2019.
Percutaneous Coronary InterventionFor PCI, use of second-generation drug-eluting stents with thinner struts and more biocompatible or even biodegradable polymers is recommended, because they show similar or even greater effectiveness and superior safety, compared to first-generation stents.
The recommendation of fully absorbable stents is rather surprising because no data are available on their long-term behavior. While we do not wish to make an assessment, it should be emphasized that, according to the Guidelines, metal stents use is limited to patients with a contraindication for prolonged dual antiplatelet therapy. After stent implantation, recommendations for aspirin and clopidogrel use as a dual antiplatelet therapy are followed. Although available studies with the new P2Y12 receptor inhibitors (prasugrel and ticagrelor) have been carried out in patients with ACS, the Guidelines, showing common sense, dare to suggest a recommendation (IIb C) for specific high-risk situations, such as stent thrombosis (usually resulting in ACS), main artery lesions management, or diabetic patients.
Regarding functional impact assessment in coronary stenoses, use of a pressure guide to measure fractional flow reserve (FFR) is clearly advocated. In patients with FFR > 0.80, medical treatment should be continued; lesions resulting in FFR < 0.8 should be revascularized. Thus, use of FFR is recommended as a guide for the need to treat a dubiously significant stenosis.
Percutaneous Coronary Intervention vs Bypass SurgeryRecently published 5-year follow-up results from the SYNTAX study and reported findings from the FREEDOM study in diabetic patients made the present update necessary. When comparing PCI to surgery, levels of recommendation (I-III) and evidence (A-C) are replaced with an algorithm for individually tailored decision-making, based on the Heart Team concept. In fact, in clinical practice a large number of clinical, anatomical, technical, and local factors must be analyzed to assess the need for and appropriat e type of revascularization. According to the Guidelines, only in patients with 1-or 2-vessel disease not involving proximal anterior descending artery could PCI be used without prior involvement of a Heart Team. In the currently proposed algorithm for the selection of a revascularization method, a number of anatomical factors and surgical risk evaluation are considered, and some clear-cut indications are stated. According to such indications, a specific therapeutic option can be selected, based on site-specific protocols. Any revascularization decision should take into account the number of vessels showing proximal involvement (including anterior descending artery), SYNTAX score, and surgical risk. This attractive approach is significantly limited by a large interobserver variability in SYNTAX score estimates.8 According to the new approach, most patients with a 3-vessel disease, low or intermediate surgical risk, and SYNTAX score ≥ 23 should be transferred to surgery directly (depending on local infrastructure availability). Some specific cases should be discussed in a case conference if significant comorbidities or special circumstances are present. The same approach is used for main artery involvement; according to the algorithm, Heart Team discussion should be used for patients with SYNTAX score ≤ 32 or bifurcation involvement. On the other hand, a surgical option should be selected for patients with 3-vessel disease and main artery involvement, low surgical risk, and SYNTAX score ≥ 32. Finally, PCI should be preferred in patients with ostial involvement and high surgical risk.
Chronic Total OcclusionThe Guidelines barely address the indication of revascularization for such lesions, saying only that this “needs to be discussed” in patients with refractory angina or large ischemic areas. Currently available evidence to support this indication comes from a meta-analysis of 13 observational studies. Two randomized studies are currently underway.
In patients with chronic total occlusion, PCI is technically challenging, does not guarantee a successful procedure in a substantial number of patients, and consumes large quantities of resources, especially time. Thus, only carefully selected patients should undergo PCI, and the procedure should only be performed by experienced interventional specialists.
Practical Considerations on RevascularizationIn our setting, the present Guidelines provide a unique opportunity to disseminate the multidisciplinary Heart Team decision-making methods, as previously done for transcatheter aortic valve implantation. Nowadays, 1-dimensional decision-making approaches are inadequate. Clinical cardiologists and imaging specialists, along with surgeons and interventional cardiologists, should work together in case conferences to reach a consensus on the optimal therapeutic option for each patient.
This is undoubtedly an appealing and optimal approach. However, using it in daily clinical practice is challenging because only patients with a preliminary indication of surgical therapy are discussed in medical rounds. Patients who received a straight PCI after a diagnostic catheterization (ad-hoc angioplasty) are not usually included. More flexible organizational structures and physical or virtual proximity of all involved health professionals are clearly needed to allow unplanned Heart Team meetings to be called and to avoid unnecessary prolonged hospital stays and a substantially increased number of hemodynamic secondary procedures.
In any case, according to Primer Registro Español de Cirugía Cardiovascular del Adulto9 (First Spanish Register of Adult Cardiovascular Surgery) findings, Spain has the lowest proportion of coronary surgical procedures compared to other surgical procedures in Europe (29% in Spain vs 62% in the rest of Europe) and also the lowest proportion of surgeries to PCI procedures. This is due to epidemiological reasons (lower CAD prevalence), but also to a number of other factors, among which we must include the longstanding questions in Spain about choosing coronary surgery because of concerns about mortality rates. Nevertheless, recently published data from this European registry show that the raw mortality rate for coronary surgery in 14 (probably highly selected) Spanish centers was 3% (slightly higher than the 2010 European mean value [2.3%]), but the risk-adjusted mortality rate was 1.4% (lower than the risk-adjusted mortality in United Kingdom [1.8%]). Thus, fully competent centers do exist in our country. Maybe it is time to publicize outcomes and redistribute surgical activity, so that it increases and is concentrated in appropriate centers, rather than avoiding surgery in patients with a clear-cut indication for surgical therapy. Another reason for an excessive number of patients being referred to percutaneous revascularization is the length of surgery waiting lists. This problem should be controlled to allow for the redistribution of surgical activity.
CONFLICTS OF INTERESTNone declared.
Working Group of the SEC on the 2013 ESC Guidelines on the Management of Stable Coronary Artery Disease: Alfredo Bardají (coordinator), Fernando Worner (coordinator), Joaquín Alonso, Joaquín Barba, Juan Cosín, Javier Ortigosa, Eduardo Pinar, and Jacobo Silva.
Reviewers for 2013 ESC Guidelines on the Management of Stable Coronary Artery Disease: Fernando Arós, Gonzalo Barón-Esquivas, José Barrabés, Vicencio Barrios, Felipe Hernández, José Luis López-Sendón, Jesús Peteiro, Xavier Ruyra, and Alberto San Román.
SEC Guidelines Committee: Antonio Fernández-Ortiz (chairman), Angel M. Alonso, Manuel Anguita, Ángel Cequier, Josep Comín, Isabel Diaz-Buschmann, Ignacio Fernández Lozano, José Juan Gómez de Diego, Manuel Pan, and Fernando Worner.
A list of all contributors is provided in the annex.