Keywords
INTRODUCTION
Increasingly aged population, early cancer diagnosis and new therapies that increased survival of patients with malignancies, favour the appearance of cancer patients who simultaneously have a cardiomyopathy requiring surgery. The benefits, principally in terms of long-term survival, and risks, have not yet been clearly established.1-5
In our population, we evaluate the following characteristics of patients with cancer undergoing cardiac surgery with extracorporeal circulation (ECC) indicated for a cause other than the tumor: type of procedure required, morbidity and mortality, long-term survival, and incidence of tumor recurrence.
METHODS
From January 2003 to May 2007, 89 (4.2%) of 2146 consecutive patients operated with ECC in our center had a cancer diagnosis. After full preoperative oncologic evaluation (with tumor staging and survival prognosis), the decision on cardiac surgery was taken only if estimated short- or medium-term survival with respect to the cardiomyopathy that established the indication was less than that associated with the neoplasia. Depending on the tumor stage, we classified patients in 2 groups:
- Group A: patients with active cancer at the time of the intervention (33 patients). In 13 patients cancer diagnosis was recent and no anti-tumor treatment had yet been initiated. The remaining 20 patients were undergoing radiotherapy, chemotherapy, or hormone therapy, with or without previous resection surgery
- Group B: patients in complete remission (56 patients)
We matched both groups with a third (group C) consisting on 165 patients with no tumor undergoing cardiac surgery with ECC, with similar characteristics in terms of age, gender, surgery type, and comorbidity.
We retrospectively gathered data on patient demography, preoperative risk factors, characteristics of the neoplasias, cardiac surgery type and in-hospital morbidity, and mortality. During follow-up, we evaluated mortality, readmission (caused by tumor, heart, or other etiology), incidence of tumor recurrence or appearance of new neoplasia during follow-up. In this period, data were obtained through out-patient clinical review and telephone interviews with survivors or their families and/or their oncologists.
Statistical analysis was conducted with SPSS 13.0S. Preoperative variables were compared using c2 and the Mann-Whitney U test. We analyzed risk factors and actuarial survival using Cox logistic regression proportional risk model and Kaplan-Meier test. A P value less than .05 was considered significant. To adjust the Cox model, we considered all preoperative variables found significant in the univariate analysis (chronic obstructive pulmonary disease [COPD], left ventricular dysfunction, interval between cancer diagnosis and surgery <2 years, and group according to tumor stage), and those with P<.15 (peripheral vascular disease), as well as age and gender.
RESULTS
Clinical characteristics, preoperative risk factors, and surgical procedure type in groups A, B, and C appear in Table 1 and the distribution of neoplasias, in Table 2.
Surgical Mortality, Postoperative Complications, and Hospital Stay
With a median interval between cancer diagnosis and surgery of 60 (1-480) months, in-hospital mortality of patients affected by some type of tumor was 4 (4.5%) patients (2 in group A and 2 in group B), all due to cardiac causes. In group C, in-hospital mortality was 5.4% (9 patients: 6 due to cardiac causes, 1 neurologic, 1 septic, and 1 respiratory). In the postoperative period, some type of complication appeared in 36% of patients with malignancy neoplastic disease (39.9% in group A and 33.9% in group B) and in 32.1% of patients in group C. Frequency and distribution are detailed in Table 3.
The statistical study has not enabled us to identify risk factors of surgical mortality in patients affected by cancer (groups A and B) and only preoperative kidney failure is identified as a risk factor for complications in the postoperative period (P=.004; odds ratio [OR] = 9.1; 95% confidence interval [CI], 1.8-46.4).
Mean hospital stay of patients with malignancy neoplastic disease was 12.2 (9.2) days, with intensive stay care of 3.6 (6.9) days. We did not find significant differences in length of hospitalization and stay in intensive care of this group of patients by comparison with group C (12.1 [11.3] and 4.2 [9] days, respectively) nor when comparing groups A and B (mean length of hospitalization, 13 [11.8] and 11.7 [7.2] days; stay in intensive care, 4.4 [10.2] and 3.1 [3.8] days, respectively).
Postoperative Mortality, Tumor Recurrence, and Readmission
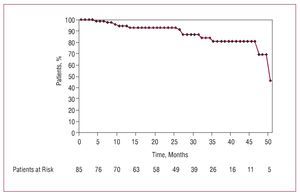
We conducted a follow-up of 100% of the patients, with medians of 22 months for group A patients, 29.5 months for group B, and 36 months for group C (interval, 1-52 months). Actuarial survival of patients with malignancy neoplastic disease is shown in Figure 1; 12 died: 8 due to a progression of the cancer (with a mean interval from the intervention of 25.2 [15.9] months), 3 due to cardiac causes, and 1 of stroke. Deaths due to cancer were secondary to recurrence of a uterine tumor in complete remission 5 years earlier (1 patient) or progression of active neoplasias (7 patients) located in bladder (2), stomach (2), lung (1), breast (1), and colon (1); 16 (18.8%) patients had a recurrent tumor, with a mean time of reactivation of 9.3 (9.1) months after surgery: 9 belonged to group A and 7 to group B. In the first group, the mean time of reactivation was 6.7 (6.9) months and in the second, 12.1 (10.3) months. Of 9 patients belonging to group A with a recurrent tumor, in 4 patients the cancer was diagnosed in the 3 months prior to surgery. In 2 patients new tumors were diagnosed during follow-up.
Figure 1. Actuarial survival of 89 patients affected by cancer, whether active or in complete remission, during follow-up after cardiac surgery with extracorporeal circulation.
In group C, 14 patients died (10 of cardiac causes, 3 of neumopathies, and 1 of neoplasia). We found a significant difference (P=.002) in cause of mortality in this period between groups A (active tumor) and C (control) in favor of the tumor etiology in the former (77.7% and 8.3%) by comparison with cardiac causes (2.2% and 71.4%) that we did not find between groups B and C.
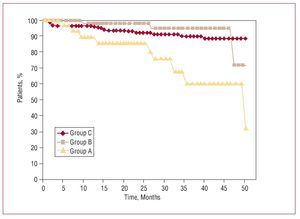
Survival during follow-up in groups A, B, and C is shown in Figure 2. In group C, only age (P=.0004) was a risk factor for mortality in follow-up (hazard ratio [HR] = 1.19; 95% CI, 1.08-1.32). Risk factors for reduction of survival in patients with cancer appear in Table 4. No factor is significantly associated with tumor recurrence.
Figure 2. Actuarial survival after cardiac surgery with extracorporeal circulation as a function of the stage of the cancer (active in group A and in complete remission in group B) and comparison with the control population (group C).
In the group of patients affected by a tumor, the type of valvular prosthesis implanted (mechanical or bioprosthesis) does not influence in-hospital mortality (6.1% and 0%) or during follow-up (13% and 17.4%) nor in the appearance of postoperative complications (30.4% and 32.6%), recurrence of cancer (21% and 21.4%), or the appearance of new tumors (4.8% and 0%).
The number of readmissions was significantly greater in patients with malignancy neoplastic disease (10.5%) (9.6% in group A and 11.1% in group B) than in group C (4.4%). Cause was cardiac in 42.8% of group C patients, whereas in groups A and B 87.5% of the readmissions were due to the tumor or complications derived from it.
DISCUSSION
Controversy about the suitability of cardiac surgery in patients with cancer, especially if they are not in complete remission, is increasing frequent in daily clinical practice. As no objective reasons exist to justify the greater mortality in these patients, the interest of the procedure centers on perspectives for long-term survival and the risks due to ECC6 use through direct alteration of the immune system,1,7-9 can exacerbate the tumor and favor dissemination10 and/or recurrence.
Since the 1990s, studies about cancer patients undergoing cardiac surgery have been published.1,2,6,7,11 Some exclude recently diagnosed or untreated1 cancers and others refer exclusively to the association of cardiac surgery and the simultaneous or deferred surgical excision of pulmonary tumors.4,7,8 A third group describes the results of surgical treatment of cancers that affect the heart as primary tumor or metastasis and that need ECC for resection.9,12 Finally, one last group includes patients affected by tumors with special characteristics such as hematologic malignancies.3,4,11 Publications are few and far between and the series are limited and heterogeneous due to the low number of patients operated. It is difficult to avoid the risk of selection bias and, moreover, the different origins, stages, and degrees of differentiation and propagation of the tumors make the comparison of results difficult.
Our study group includes all patients affected by a neoplasia presenting over a 4-year period, after diagnosis of extension and survival estimation by an oncologist. Surgery was accepted only if the estimated prognosis of survival time in cancer patient was greater than the estimated for the cardiomyopathy that conditioned indication for surgery. Patients with metastasis did not undergo surgery. In 6 patients, diagnosis of the tumor and cardiac disease was almost simultaneous so surgery was performed prior to treating the cancer. In no patient were cardiac surgery and excision of the tumor performed during the same intervention.
The number of patients with isolated cardiac surgery is low (17% vs 34% in the general population in our area). This may be due to a selection bias, inevitable on our part, as only patients with clinical angina non elegible for percutaneous treatment thru angioplasty-stent are referred for surgical intervention. Therefore, the low number of patients operated without ECC (2 patients) prevents us from comparing morbidity and mortality, survival, and tumor recurrence with patients operated with ECC13,14 consequently they have been excluded from the study.
In-hospital mortality (4.5%) is comparable with that of other series (4.1%-17%),1,3-6 similar to that expected according to the Euroscore scale and in no case a consequence of the tumor. Thus, neither in patients with malignancies in treatment or recently diagnosed can this be used as an excuse to exclude them from surgery.
With regard to morbidity, no postoperative complication seems significantly linked to cancer, whether active or in complete remission. Hematologic malignancies constitute a special group of tumors associated with greater mortality and incidence of hemorrhagic complications,1,3,4,11 infections, and mediastinitis after ECC.2-4 In these patients, various authors advise careful preoperative evaluation and the use of techniques without ECC or valve bioprostheses. The 10 patients affected in our series by hematopathy did not present greater mortality or incidence of infectious, or hemorrhagic complications.
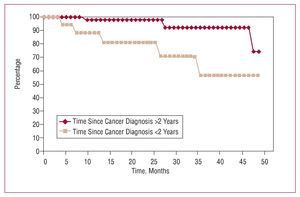
As in other series,1-3,6 long-term survival is directly related, firstly, with the cancer (which caused 8 deaths) and secondly with the cardiomyopathy, especially in patients with preoperative left ventricular dysfunction (3 deaths). Although survival is significantly reduced in patients with active cancer (group A), the distance between the curves in Figure 2 does not really become important until 24 months of follow-up, even when compared with survival in patients without known tumors (group C). Consequently, it seems inappropriate to exclude cardiac surgery in patients with cancer (even in the active phase) solely as a function of expected survival because, although less, results seem acceptable. As in other studies,6 survival falls if the interval between cancer diagnosis and surgery is <2 years (Figure 3). Although studies exist that indicate metastasis inhibits anticoagulant therapy.9,15 Furthermore, we have found no significant differences in risk of hemorrhage, survival, tumor recurrence, metastasis, and/or appearance of new tumors as a result of its use.
Figure 3. Survival of the patients with cancer (groups A and B) as a function of the interval between cancer diagnosis and cardiac surgery with extracorporeal circulation. Survival is reduced in patients with an interval <2 years.
During follow-up, mortality of neoplasic etiology does not appear to be related with use of ECC, as only 1 of 8 deaths is identifiably due to tumor reactivation; the rest are due to the evolution of active cancers. Although the incidence of tumor recurrence in group A is double that in group B (33.3% and 15.5% respectively), in group B recurrent tumors are late and their timing is difficult to relate with immune system alterations associated with ECC. Similarly, readmissions are more frequent among patients affected by cancer, both for need for treatment and the appearance of complications secondary to the tumor.
Limitations of the Study
As this study is retrospective with a limited number of patients, results should be interpreted with caution. Groups A and B are heterogeneous in origin, extension, and stages of the cancers and there is an uncontrollable selection bias that reduces the number of coronary patients by comparison with the general population in our area. This complicates the construction of a control group composed of patients operated without ECC to determine whether the technique is efficient in reducing recurrence and prolonging survival.
CONCLUSIONS
We have found no significant increase in morbidity and mortality of cardiac surgery with ECC in cancer patients, even in an active stage, by comparison with the normal population in our area.
It seems that the survival of cancer patients who undergo cardiac surgery is more closely related with the progression of the tumor than the surgical procedure. Medium-term survival is acceptable, although less in patients with an active cancer at the time of the intervention and in those with <2 years between cancer diagnosis and surgery. Rates of readmission are greater than in the general population and are principally due to need for treatment of the cancer and/or complications arising from it.
Individual evaluation of each case should consider both the tumor stage and chances of complete remission (even in active cancers) before discounting heart surgery.
Wider-ranging studies are needed to enable comparison of results of morbidity and mortality, and survival with and without the use of ECC to identify its influence on these variables and on long-term recurrence.
ABBREVIATIONS
CI: confidence interval
COPD: chronic obstructive pulmonary disease ECC: extracorporeal circulation
HR: hazard ratio
LVEF: left ventricular ejection fraction
OR: odds ratio
SEE EDITORIAL ON PAGES 349-51
Correspondence:
Dra. Yolanda Carrascal.
Servicio de Cirugía Cardiaca. Hospital Universitario de Valladolid. Avda. Ramón y Cajal, 5. 47003 Valladolid. España.
E-mail: ycarrascal@hotmail.com
Received July 10, 2007.
Accepted for publication November 15, 2007.