Progress in medical therapy wouldn’t be possible without the contribution of the scientific community. Several randomized controlled trials have led to our current guidelines. Specifically, COMPANION and CARE-HF trials involved a turning point for cardiac resynchronization therapy, which became well recognized for the treatment of heart failure patients with QRS≥120ms, ejection fraction≤35%, and sinus rhythm to reduce hospitalizations and all-cause mortality. New indications were then established for atrial fibrillation, pacemaker-dependent, and mildly symptomatic patients, but new challenges should be addressed, namely reducing complication and nonresponder rates. To achieve this, further studies and new implant techniques are under investigation.
Keywords
Heart failure (HF) is one of the most disabling, deadly, and costly cardiovascular diseases in western countries. Over the last decades many advances in pharmacological treatment have increased life expectancy in HF patients and have mitigated their HF symptoms. Nevertheless, mortality and quality of life remain a matter of concern in the vast majority of HF patients. In 1990 Dr. Mower1 introduced in clinical practice biventricular (BiV) pacing for the treatment of myocardial dysfunction associated with left bundle branch block (LBBB). Since then, there has been a remarkable evolution of cardiac resynchronization therapy (CRT), in technical development as well as in understanding of the pathophysiology and pathobiology of mechanical dyssynchrony. In addition, over the last 20 years several prospective randomized controlled trials have contributed to the establishment of the current clinical practice guidelines and broadening of the indications for CRT in the HF population. Despite this significant evolution, questions and uncertainties remain. Current goals of CRT are a) to improve the rate of patients responding to the therapy, and b) to enhance the response to the therapy in those individuals who benefit from CRT.
The present article reviews the most important trials that have led to the current indications for CRT, also pointing to patient subgroups in which CRT indication is less well established. It is worth notice that, as of today, no exclusion or contraindication to CRT has been formally established.
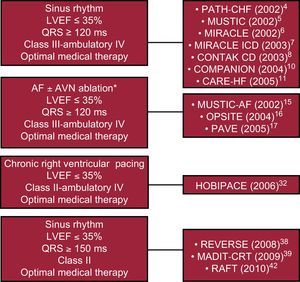
Indications of cardiac resynchronization therapy. two decades of studiesShortly after the first description of BiV pacing by Mower,1 in 1994 Cazeau et al.2 in France and Bakker et al.3 in the Netherlands reported the treatment of advanced HF by atrio-BiV pacemakers. These early reports were followed by the first 2 randomized, prospective, crossover studies: PATH-CHF4 and MUSTIC5; both studies very consistently showed clinically relevant improvements in New York Heart Association (NYHA) functional class, 6-min walk test, quality of life, and peak oxygen consumption (VO2max),. Later on, MIRACLE6 and MIRACLE ICD trials7 and the CONTAK CD8 study confirmed, in larger populations, the previously reported beneficial effects of CRT, but also convincingly demonstrated a significant effect of CRT on left ventricular ejection fraction (LVEF) accompanied by impressive reduction in left ventricular (LV) systolic and diastolic volumes and LV mass.6, 9 Finally, two landmark trials, COMPANION in 200410 and CARE-HF11 in 2005, established the benefit of CRT in terms of hospitalizations and improved survival for CRT patients. COMPANION was a three-arm trial enrolling 1520 patients in NYHA class III or IV on optimal pharmacological therapy with LVEF≤35%, QRS≥120ms, and PR interval≥150ms. Patients were randomly assigned in a 1:2:2 ratio to pharmacological therapy alone, CRT only (CRT-P), or CRT plus defibrillator (CRT-D), respectively. The primary endpoint was a composite of all-cause death and all-cause hospitalization. The median duration of follow-up for the primary endpoint was 11.9, 16.2, and 15.7 months in the pharmacological therapy, CRT-P, and CRT-D groups, respectively. Both CRT-P and CRT-D significantly reduced the risk of the primary endpoint compared with optimal medical therapy alone: 19% (P=.014) and 20% (P=.001), respectively. The risk for all-cause mortality and hospitalization for HF was reduced by 34% (P<.001) and 40% (P<.001) in the CRT-P and the CRT-D groups, respectively. All-cause mortality was reduced significantly by CRT-D (36%, P=.003) but not by CRT-P (24% reduction, P=.059). The lack of significance in the last case may have been due to the relatively short follow-up, as the mortality rate per year in the pharmacological-therapy group was up to 19%. Any remaining doubts about mortality benefits with CRT were dispelled a year later with the publication of the results from CARE-HF, which enrolled patients in functional NYHA class III/IV despite pharmacological therapy, LVEF≤35%, and QRS duration≥150ms or QRS 120–149ms associated with echocardiographic criteria for dyssynchrony. All patients in the active treatment group were implanted with a CRT-P. During a mean follow-up of 29.4 months, the risk of the primary outcome of death or HF hospitalizations was reduced by 37% (P<.001) and that of HF hospitalizations by 39% (P<.001). As in COMPANION, there were significant improvements in NYHA class and quality of life. In CARE-HF, CRT-P reduced all-cause death by 36% (P<.002), similar to the reduction in COMPANION. Moreover, a predefined long-term follow-up over an average of 37.4 months with all-cause death as primary endpoint reported a significant 40% reduction with CRT-P (P<.0001). There was also a report of reduced risk of sudden cardiac death with CRT-P in CARE-HF.12 Conceivably, improved cardiac function with CRT could be expected to reduce the incidence of serious arrhythmias and thus the risk of sudden death, but this issue remains unresolved. A 2006 metaanalysis of randomized controlled CRT trials in HF including about 3000 patients reported a 29% reduction in all-cause mortality with CRT.13
These favorable and consistent results are well summarized in the most recent recommendations of European Society of Cardiology guidelines14: patients who remained in NYHA class III-IV despite optimized pharmacological therapy, with low LVEF (≤35%), in sinus rhythm, and with a QRS duration≥120ms (class I indication, level of evidence A).
Patients in atrial fibrillationHF patients with atrial fibrillation (AF) usually have more comorbidities and a worse prognosis despite optimal pharmacological treatment than those in sinus rhythm. This group of HF and AF patients represents up to 30% of the overall chronic HF population, with increasing prevalence in aged patients. The greater severity of symptoms in this population can be explained by lack of atrial active filling and related atrioventricular (AV) synchrony, and irregular RR interval and relatively higher mean heart rate, both of which significantly shorten ventricular filling time.
The first multicenter randomized study (MUSTIC-AF)15 included 64 patients in persistent and permanent AF patients with an LVEF<35% and NYHA class III. However, only 37 patients completed the follow-up. Notably, 63% of patients underwent an AV node ablation. During a period of 6 months, patients were randomized in two 3-month crossover periods comparing univentricular right ventricular (RV) pacing and BiV pacing. The primary end-point was the 6-min walk distance; the secondary end-points were VO2max, quality of life, hospital admissions for HF, mortality and patient's preferred pacing mode. There was no statistically significant difference between the modalities in the intention-to-treat analysis, but in the 37 patients who completed the efficacy test there was a significant improvement of the 6-min walk distance and VO2max with BiV pacing. The majority of these patients (84.6%) preferred the period corresponding to the BiV pacing phase. The high patient drop-out rate limited the statistical power of this trial.
A second prospective randomized trial (OPSITE)16 included 56 patients with permanent AF divided in 2 subgroups: LVEF>40% and absence of LBBB vs LVEF<40% and LBBB. All patients underwent an AV node ablation and were implanted with a BiV device. Patients were also randomized in a 3-month crossover design comparing RV pacing, LV pacing, and BiV pacing during a follow-up period of 12 months. The primary end-points were quality of life and exercise capacity. All patients improved their quality of life and exercise capacity from baseline, but up to 25% of patients had better results with RV pacing than with LV or BiV pacing, showing a dominant effect of heart rate control over resynchronization. There was no significant statistical difference in the subgroup analysis.
Subsequently, the PAVE trial17 compared chronic BiV pacing to RV pacing in patients undergoing ablation of the AV node for management of permanent AF with rapid ventricular rates. The 148 patients with an LVEF of 0.46±0.16 and NYHA class II or III (on average, without significant differences between groups) were randomized to receive a BiV pacing system (n=103) or RV pacing system (n=81). The primary endpoint was the change in the 6-min walk test at 6 months postablation. The secondary endpoints were changes in quality of life and LVEF. At 6 months postablation, patients treated with cardiac resynchronization had significant improvement in 6-min walk distance (31% from baseline, 82.9±94.7 m), compared to patients receiving RV pacing (24% above baseline, 61.2±90 m) (P=.04). There were no significant differences in the quality of life parameters. The LVEF in the BiV group (0.46±0.13) was significantly greater in comparison to patients receiving RV pacing (0.41±0.13; P=.03). Notably, patients in the BiV pacing group with an LVEF≤45% (37 patients in the BiV pacing group and 39 in the RV pacing group) or with NYHA class II/III symptoms (90 patients in the BiV pacing group and 61 in the RV pacing group) had greater improvement on the 6-min walk distance compared to patients with normal systolic function or class I symptoms.
More recently, several large observational registries have reported positive results on resynchronization in AF patients with LV dysfunction and LBBB.18, 19, 20, 21, 22, 23, 24 Gasparini et al.25 published the prospective observational study with the largest sample (673 patients) and longest follow-up (4 years) comparing permanent AF patients (162) and sinus rhythm patients (511). The benefit from resynchronization was similar for both groups in terms of functional capacity, reverse remodeling, and LV function. Of note, only the patients who underwent an AV node ablation showed an improvement of LVEF (P<.001), LV end-systolic volume (P<.001), exercise capacity (P<.001) and a higher proportion of responder patients at 12 months (68% in the ablated group compared with only 18% in the nonablated group).
Despite the positive results coming from this and other studies,26 the need for AV node ablation in this population is still very controversial, pointing towards the need for multicenter, randomized studies.
As a result, the indication for resynchronization in the last international guidelines27 is class IIa, with a B level of evidence if an AV node ablation is performed and a C level of evidence if there is ≥95% of BiV pacing without AV node ablation.
Pacemaker-dependent patientsAt the present time there is sufficient evidence to assert the negative effect of RV-apex pacing on synchrony in patients with and without LV dysfunction.28, 29 This type of stimulation causes an electrocardiographic LBBB pattern that generates a dyssynchrony similar to the one in spontaneous LBBB and a delay in LV contraction. From small-scale experimental studies30, 31 to the first randomized trial in 2006 (HOBIPACE)32 and the latest observational studies,33, 34, 35, 36 all have consistently showed positive results following addition of an LV lead for pacemaker-dependent patients with moderate to severe LV dysfunction, severe HF symptoms (NYHA class III-IV), and long-term apical RV pacing. While most of the studies showed significant improvements in LVEF, LV end-diastolic diameter, LV end-systolic diameter and functional class, reductions in hospitalization rate, mortality and morbidity have not been reported so far.
In patients with normal systolic function, Yu et al.37 recently reported that conventional RV apical pacing resulted in adverse LV remodeling and a reduction in LVEF; these effects were prevented by BiV pacing. There are several limitations of this study. The sample was small, and the study was not powered to detect significant differences in clinical events. However, the study was designed with adequate power to test for the expected differences between the 2 pacing groups with respect to LV systolic function and LV volume. The success rate for implantation of the BiV pacing system was 92%, which is lower than that for conventional dual-chamber pacing but similar to that for pacemakers implanted in patients with HF. Large, prospective, randomized, controlled trials in different patient populations with indication for conventional RV pacing are still ongoing and results are expected shortly.
Mildly symptomatic patientsOver the last 2 years, several landmark studies that included less symptomatic patients have been published. The first, the REVERSE38 trial, enrolled 610 patients in NYHA class I or II with a QRS≥120ms and LVEF≤40%. All patients received a CRT device (with or without defibrillator) and were randomly assigned to active CRT or to control. The primary analysis was carried out after 12 months of follow-up, with the European population (n=262) remaining in the trial and further analyzed after 24 months. REVERSE used a clinical composite endpoint, scoring patients as worsened, unchanged, or improved. As it is difficult to show clinical improvements in NYHA class I patients, the criterion for success was predefined as the proportion of patient worsening only. This primary endpoint was not met at 12 months despite a significant difference in favor of CRT in the distribution of patients who worsened, remained unchanged, or improved; CRT also reduced the risk of death or HF hospitalizations at 12 and at 24 months with a 53% reduction (P=.03) at 12 months and 62% (P=.0003) at 24 months. Both LV function and remodeling improved significantly with CRT. Although there was a nonsignificant trend toward lower all-cause mortality with active treatment, the trial was not powered to show differences in all-cause death in this population where mortality rates were low.
Very similar results were reported by the larger (n=1820) MADIT-CRT trial.39 Included patients had NYHA class I or II HF, LVEF≤30% and QRS≥130ms. Patients were randomized in a 3:2 ratio to receive CRT and defibrillator or a defibrillator alone. Although physicians were not blinded to treatment assignments, the members of the assessment committee were unaware of such assignments. During an average follow-up of 2.4 years, CRT reduced the risk of the primary endpoint of all-cause death or non-fatal HF events by 34% (P=.001), although there was no reduction in all-cause death alone. As in REVERSE, CRT improved LV function significantly at 12 months. Although mortality was not significantly reduced in either study, the consistency of the other clinically relevant findings and the improvements in LV function argue for benefits of CRT in NYHA class II patients. A subanalysis of MADIT-CRT showed the benefits from CRT were driven by those patients with QRS interval>150ms and LBBB.40
Some evidence of possible benefit on all-cause mortality or hospitalization for HF in these healthier patients (21.5% of the study population was in class I or II) was provided by a post hoc analysis of the CARE-HF study, which showed that the outcome of these patients was similar to those in the overall trial population.41
Definitive evidence was provided by the RAFT study,42 a multicenter, double-blind, randomized study that enrolled 1798 patients to either implantable cardioverter-defibrillator (ICD) or ICD with CRT. The inclusion criteria were NYHA class II (80%) or III, LVEF≤30% and QRS≥120ms or paced QRS≥200ms. The primary endpoint was death from any cause or HF leading to hospitalization. There was a reduction of the relative risk for all-cause death of 25% in patients with CRT-D compared with those with ICD only. The risk of cardiovascular death and hospitalization for HF were also significantly reduced. Similar to MADIT-CRT, a greater benefit was found in patients with QRS≥150ms.
These findings led to the last update in 2010 of the European guidelines43 for device therapy in HF, which indicate the CRT for patients in NYHA class II, LVEF ≤35%, sinus rhythm, and a QRS width of≥150ms (recommendation class I, level of evidence A) (Figure).
Figure. Broadening of recommendations for cardiac resynchronization therapy in relation to the main trials. AF, atrial fibrillation; AVN, atrioventricular node; LVEF, left ventricular ejection fraction. * ≥95% of biventricular pacing is needed if atrioventricular node ablation is not performed.
Patients with narrow QRSThere is a significant heterogeneity in the definition of wide vs narrow QRS. Indeed, some authors considered “narrow” a QRS duration<150ms, whereas others assigned a QRS cut-off at 130ms to dichotomize narrow/wide, and a few others set the QRS duration for narrow QRS<120ms (which clearly indicates normal QRS duration). Thus, it is important to recognize these differences for proper comparison between studies.
Several small single-center studies have addressed the efficacy of CRT in HF patients with a narrow QRS complex (<120ms) compared to wide QRS complex. In an early study published in 2003,44 38 patients with a wide QRS complex and 14 patients with a narrow QRS complex were compared. All had echocardiographic signs of interventricular delay of more than 20ms and intraventricular dyssynchrony (posterolateral LV wall activation delay>interval between QRS onset and beginning of transmitral filling). After 6 months of CRT, improvement in NYHA functional class, 6-min walk distance, LVEF, LV end-systolic diameter, LV end-diastolic diameter, and mitral regurgitation were observed to a similar degree in both groups. In another study45 of 102 HF patients with functional NYHA class III or IV, standard deviation of the tissue Doppler imaging time to peak systolic velocity in 12 LV segments (>32.6ms) was used to depict subjects with mechanical LV dyssynchrony among 51 patients with a narrow QRS. A reduction in LV end-systolic volume was observed in the narrow and wide QRS patient group 3 months after CRT implantation; improvements in NYHA functional class, maximal exercise capacity, 6-min walk distance, LVEF, and mitral regurgitation were also detected. In contrast, withholding CRT for 4 weeks resulted in loss of echocardiographic benefits. In both groups, LV reverse remodeling was determined to a similar extent by the degree of baseline mechanical dyssynchrony. In a similar study,46 33 consecutive patients with a narrow QRS complex were prospectively compared with 33 consecutive patients with a wide QRS complex. Inclusion criteria were NYHA functional class III or IV, LVEF≤35%, and signs of LV dyssynchrony on tissue Doppler imaging (maximum delay between peak systolic velocities among the four LV walls≥65ms). No significant relationship between baseline QRS duration and LV dyssynchrony was observed, and improvements in clinical symptoms (NYHA functional class, 6-min walk distance, quality of life) or LV reverse remodeling (LV end-systolic volume reduction, increase in LVEF) were similar in both groups after 6 months of CRT. A meta-analysis of the 3 previously47 mentioned studies confirmed an improvement by CRT in mean LVEF and NYHA functional class in HF patients with narrow QRS complexes. These small pilot studies demonstrated that patients selected on the basis of echocardiography-based dyssynchrony criteria may benefit from CRT independently of QRS duration.
Worth mentioning is the observational, longitudinal study by Gasparini et al.48 which confirmed the findings of improved functional capacity and LV function in patients with narrow QRS (≤120ms), who were, however, not preselected according to any echocardiographic dyssynchrony criteria.
Although the findings were consistent in all of these small studies, all were limited by lack of hard endpoints, small sample size, and short duration of follow-up. The initial enthusiasm for a possible benefit of CRT in patients with narrow QRS was subsequently tempered by the outcome of multicenter trials that followed.
Two prospectively designed, yet moderately large, studies in patients with advanced HF and normal QRS complex have been completed: the ReThinQ49 and ESTEEM-CRT50 trials; both studies missed the primary endpoint and turned out to be negative. RethinQ enrolled 172 HF patients with NYHA class III, LVEF≤35%, QRS interval<130ms, and evidence of mechanical asynchrony as measured by echocardiography. All patients received a defibrillator and were on optimized medical therapy. In a small, prespecified subgroup with QRS of 120 to 130ms, significant improvements in VO2max were observed with CRT. Despite several limitations of the RethinQ study, the multicenter ESTEEM-CRT trial confirmed the ReThinQ results. ESTEEM-CRT evaluated the effects of CRT in HF patients with a narrow QRS and signs of mechanical dyssynchrony. Inclusion criteria were LVEF≤35%, QRS≤120ms, NYHA functional class III, and mechanical dyssynchrony (defined as the standard deviation of time to peak velocity of 12 segments>28.7ms). After 6 months of CRT, no improvements in VO2max, LVEF, or LV end-systolic volume were observed; in contrast, subjective measures such as quality of life score and NYHA functional class significantly improved, suggesting a possible favorable effect of CRT on diastolic function. The later is considered the most important determinant of HF symptoms.51 Major limitations of ESTEEM-CRT include its nonrandomized, single-arm, unblinded design; hence, subjective measures such as functional class improvement are highly susceptible to placebo effect. Furthermore, the same general concerns apply as for RethinQ. On one hand, the tissue Doppler imaging criteria employed may not have depicted patients suitable for CRT. More importantly, however, the single-arm design, low patient number, and short follow-up period limit the validity of the study to assess the long-term effects of CRT on LV remodeling and (eventually) morbidity and mortality. Moreover, in contrast to single-center studies, interobserver variations in the assessment of dyssynchrony may be considerably higher across different centers in multicenter trials, which may have prevented uniform selection of appropriate patients. Based upon the results of current trials, CRT should not be used in patients with normal QRS duration. An ongoing trial, EchoCRT,52 with more than 1000 patients with QRS<130ms and mechanical dyssynchrony, evaluates CRT effect on combined primary endpoint of all-cause death and HF hospitalizations. The trial is expected to report in 2013–2014.
“Ablate and pace” for permanent atrial fibrillation patientsLast year the results of the Ablate and Pace for Atrial Fibrillation53 trial were published. The study included patients indicated for AV node ablation because of severe symptomatic permanent AF. The primary endpoint was a composite of death due to HF, hospitalizations due to HF, or worsening of HF. The 186 patients were randomized to BiV pacing (97 patients) or RV pacing only (89 patients). All participants received a CRT device and underwent an AV node ablation, thereby excluding the influence of the tachyarrhythmia in the patients’ symptoms for both groups. The average LVEF was 0.38±0.14 in the CRT group and 0.37±0.14 in the RV pacing group. The median follow-up was 20 months. Although the primary endpoint was reached in the BiV group with a significantly lower incidence of death, hospitalizations, and worsening HF (11% of incidence in comparison with 26% in the group with RV pacing only), there was no significant difference in total mortality between the 2 groups.
This is the first trial to compare CRT and RV pacing in patients with an “ablate and pace” indication for AF. In spite of the nonsignificant results in mortality, its positive clinical results can encourage the initiative for more studies in this direction.
Hypertrophic obstructive cardiomyopathy patientsVery recently, Berruezo et al.54 observed the effects of LV pacing in 9 patients with hypertrophic obstructive cardiomyopathy and significant LV pressure gradient (≥50mmHg). The best settings configuration was BiV in 6 patients who not only showed significant improvements in clinical parameters (functional class, 6-min walk test and quality of life) but also a progressive decrease in LV pressure gradient from 74±23mmHg at baseline to 28±17mmHg after 1 year and a significant reduction of LV mass. Although the study population was too small and larger randomized controlled trials are required, these promising results tell us that BiV pacing could be considered when surgical or ablative treatment are contraindicated or rejected by the patient.
Contraindications of cardiac resynchronization therapyTo date, there is no formal contraindication to CRT. However, great caution should be taken in particular clinical settings. There is progressive evidence of limited CRT benefit in acutely decompensated patients or catecholamine-dependent patients. In contrast, ambulatory NYHA class IV patients are formally eligible for CRT; however, the COMPANION study has proven to have a limited benefit for hospitalization rate but not for survival.
The presence of extensive scarring of lateral wall may be considered a relative contraindication. Although no formal study has prospectively addressed this specific condition, several retrospective small studies have reported lack of significant benefit in patients with large scars over the free wall of LV.
Finally, the presence of a right bundle branch block (RBBB) is not considered a contraindication, but the benefit of CRT still needs to be proved as all clinical trials have underrepresented patients with LV dysfunction and RBBB. Some studies have shown the presence of mechanical LV dyssynchrony in RBBB patients who may benefit from CRT,55 but more research is needed.
What might be the future?The idea that the lateral wall is the target for the position of the LV lead is being challenged by some groups. Recently, Derval et al.56 proved, in a nonischemic population, that pacing at the best LV site (either endocardially or epicardially through the coronary sinus and determined by the best LV output measured invasively) was associated acutely with twice the improvement in LV contractility. Of note, no ventricular region could be correlated to the best pacing site. Therefore, the best pacing site to deliver CRT appears to be patient-specific; an individually based approach to pacing at the best possible location proved to be superior to other pacing strategies (pacing from within the coronary sinus, at the lateral wall, or at the most delayed wall region identified by myocardial strain measures).
However, to conceive a patient-specific approach for LV lead positioning, some technical development is needed. More targeted delivery of pacing can be achieved with the use of an endocardial LV lead. This avoids the limited choice of placement, the phrenic nerve stimulation commonly encountered with epicardial leads, and eventually multiple LV pacing sites. Recent preclinical work simulating different clinical settings has provided the first evidence that LV endocardial pacing is superior to epicardial pacing.57 Indeed, in the most typical LBBB dog model, endocardial-BiV pacing more than doubled the degree of electrical resynchronization and increased the benefit for contractility and stroke work by 90% and 50%, respectively, compared with more traditional epicardial BiV pacing. Even in a dog model with myocardial infarction or in which chronic rapid ventricular pacing added to LBBB determined severe HF, endocardial pacing significantly increased LV contractility compared to epicardial pacing. The mechanism by which endocardial pacing is superior to epicardial pacing is not fully elucidated although some hypotheses may be generated. LV endocardial pacing site is more natural, follows intrinsic activation, and produces a more homogenous spread of activation than epicardial pacing. The difficulty of endocardial pacing lies in access to the LV cavity. So far, most endocardial leads have been placed with a transseptal approach that accesses the LV cavity by passing from the right atrium through the left atrium. More recently, leadless pacing has been proposed. This approach has the potential advantage to simplify implantation procedures, to improve access to CRT in patients with unfavorable coronary sinus anatomy, and eventually to greatly increase the ability to pace children. Use of ultrasound or induction transfer of energy58 to a receiver electrode has been recently reported. This technology is currently under investigation in the Wireless Endocardial CRT study, a prospectively controlled study designed to evaluate the safety and feasibility of this novel pacing modality.
ConclusionsCRT has represented a revolution in the treatment of HF. Modern goals of CRT are to enhance response to CRT in those patients who respond to the therapy as well as to reduce the proportion of patients not responding to therapy. In this regard, a better pathophysiological understanding of mechanical dyssynchronopathy, thus integrating molecular, electrical, and mechanical aspects, may help us to deliver “personalized” resynchronization therapy adapted to each patient.
Conflicts of interestNone declared.
Received 19 February 2012
Accepted 24 February 2012
Corresponding author: Divisione di Cardiologia, Fondazione Cardiocentro Ticino, Via Tesserete 48, CH-6900, Lugano, Switzerland. angelo.auricchio@cardiocentro.org