The number of important articles published over the last year in the areas of cardiac electrophysiology, clinical arrhythmology, and cardiac pacing is so high that it is quite impossible to summarize the extensive information they contain in this article. Under this premise, we have attempted to select the contributions that, in our view, have had the greatest impact for the clinical cardiologist and have summarized their content as much as possible. We apologize for the necessary omission of many studies of considerable clinical importance.
ADVANCES IN THE TREATMENT OF ATRIAL FIBRILATIONCatheter AblationA registry of the European Society of Cardiology provides a view of atrial fibrillation (AF) management and the outcome of ablation therapy for this condition in Europe.1 The success of this procedure at 1 year of follow-up was 41% without drugs and 73% with drugs, with a major complications rate of 1.7%.
Several studies have been unsuccessful in demonstrating that approaches beyond pulmonary vein isolation increase the rates of favorable outcome. These studies have found that performing additional ablation lines, fractionated electrogram ablation, or ablation on dominant frequency areas does not provide further benefits.2,3 Moreover, in groups undergoing additional ablation, the procedure was longer and patients had a higher risk of complications. Therefore, pulmonary vein isolation is currently the most effective procedure for the treatment of AF.
However, the effectiveness of the available techniques in achieving permanent transmural isolation is limited and efforts are being focused on technological advances to optimize the procedure. Magnetic resonance evaluation of post-procedure atrial fibrosis rarely shows complete scarring in the areas intended for ablation. Although this feature does not seem to be associated with recurrence, the overall baseline fibrosis and fibrosis that was not covered by the ablation scar were found to be associated with recurrent AF.4 The use of contact force-sensing catheters is reported to create more effective and persistent ablation lesions that result in better outcomes over follow-up.5 Cryoenergy ablation has improved with the incorporation of second-generation cryoballoons, yielding results similar to those of radiofrequency ablation.6 Ablation using balloon-catheters with laser energy has shown acceptable efficacy and safety profiles, although further studies with long-term follow-up are needed on this option.7
Patient Management Following AblationThe AMIO-CAT8 study found that amiodarone use during the first 3 months following ablation initially reduces rehospitalizations due to recurrence and cardioversion, but does not significantly lower the relapse rate in the long-term.
Intensive treatment for excess weight and other cardiovascular risk factors is a clear predictor of success and clinical improvement following ablation,9,10 and it is essential to control these factors before and after ablation. This is due, in part, to their influence on the atrial substrate.9
The search for methods to reduce periprocedure embolic complications continues to generate considerable interest. Uninterrupted use of warfarin rather than the strategy of bridging anticoagulation with heparin has been proposed as an effective and safe approach for patients at a high risk of embolic complications, resulting in reductions in embolisms and minor bleeding.11
Developments in Oral Anticoagulation TreatmentOne of the main contributions over the last year with regard to new oral anticoagulant agents has been the emergence of idarucizumab, the first drug to provide rapid and complete reversal of the effects of dabigatran.12
A subanalysis of the ARISTOTLE study evaluated the effects of apixaban and warfarin in patients with moderate-severe valvular disease (except those with severe mitral stenosis or a mechanical prosthesis). No differences were found regarding the incidence of stroke or systemic embolism, but apixaban was associated with a decrease in bleeding and mortality rates.13
In patients on hemodialysis, both rivaroxaban and dabigatran were associated with a higher risk of hospitalizations or death due to major bleeding than warfarin treatment.14
The ORBIT-AF15 study showed that bridging therapy with heparin in patients with nonvalvular AF scheduled for surgery is associated with higher rates of bleeding and major events than temporary discontinuation without bridging therapy.
A registry in Taiwan16 (n = 12 935) reported the benefits of oral anticoagulation in a subgroup of patients at a low risk of embolism and bleeding: CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled]-vascular disease and sex category [female]) score = 1 (male), 2 (woman). A Danish registry17 (n = 39 400) provided further evidence that patients with lower scores on the CHA2DS2-VASc scale (0 [male], 1 [woman]) have a lower embolic risk and concurred with the Taiwanese registry in that the presence of 1 additional risk factor significantly increases the risk of embolic events. In patients with a history of intracranial bleeding, oral anticoagulant therapy significantly reduced the incidence of recurrent ischemic events and was not associated with increases in recurrent bleeding compared with discontinuation of this therapy.18
Finally, a consensus document has emerged, focused on antithrombotic therapy management in patients with AF and an acute coronary syndrome, and those undergoing a percutaneous coronary or valvular intervention.19 The document voiced some concern about triple therapy and recommended shortening it as much as possible. Prompt dual therapy or single therapy was proposed as a potential option in patients with stable coronary disease and no other risk factors.
Diet and Antiarrhythmic Drug TreatmentThe importance of diet in AF prevention has received further attention this year. In a subanalysis20 of the PREDIMED study, the Mediterranean diet supplemented with a specific amount of extra-virgin olive oil was shown to reduce the incidence of AF in patients with intermediate-high cardiovascular risk.
The role of digoxin in the treatment of AF continues to be a topic of debate. In a recent study21 (n = 2267), this drug was found to have a neutral effect on the time to symptom onset and hospitalization. Mortality was not higher in patients with heart failure but did increase in those without. The results of the RAFFAELO22 study should also be mentioned: following AF cardioversion, ranolazine use did not prolong the time to recurrence but did decrease the recurrence rate.
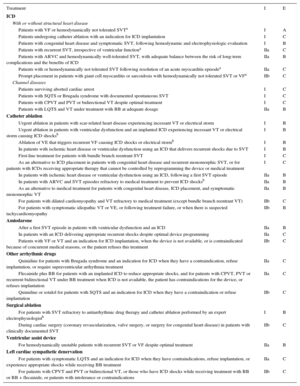
ADVANCES IN THE TREATMENT AND PREVENTION OF VENTRICULAR ARRHYTHMIANew Clinical Practice GuidelinesVery recently, the European Society of Cardiology published a new set of guidelines on the management of patients with ventricular arrhythmias and prevention of sudden cardiac death (SCD). The document was conceived as an update of the 2006 guidelines published jointly with the American Heart Association and American College of Cardiology and places greater emphasis on SCD prevention. The recommendations23 for the main antiarrhythmic therapy options for secondary SCD prevention and those for placement of implantable cardioverter-defibrillator (ICD) for primary prevention are summarized in Tables 1 and 2.
Indications for the Main Arrhythmia Therapies for Secondary Prevention of Sudden Cardiac Death in Patients With Sustained Ventricular Arrhythmia
| Treatment | I | E |
|---|---|---|
| ICD | ||
| With or without structural heart disease | ||
| Patients with VF or hemodynamically not tolerated SVTa | I | A |
| Patients undergoing catheter ablation with an indication for ICD implantation | I | C |
| Patients with congenital heart disease and symptomatic SVT, following hemodynamic and electrophysiologic evaluation | I | B |
| Patients with recurrent SVT, irrespective of ventricular functiona | IIa | C |
| Patients with ARVC and hemodynamically well-tolerated SVT, with adequate balance between the risk of long-term complications and the benefits of ICD | IIa | B |
| Patients with or hemodynamically not tolerated SVT following resolution of an acute myocarditis episodea | IIa | C |
| Prompt placement in patients with giant cell myocarditis or sarcoidosis with hemodynamically not tolerated SVT or VFa | IIb | C |
| Channel diseases | ||
| Patients surviving aborted cardiac arrest | I | C |
| Patients with SQTS or Brugada syndrome with documented spontaneous SVT | I | C |
| Patients with CPVT and PVT or bidirectional VT despite optimal treatment | I | C |
| Patients with LQTS and VT under treatment with BB at adequate dosage | IIa | B |
| Catheter ablation | ||
| Urgent ablation in patients with scar-related heart disease experiencing incessant VT or electrical storm | I | B |
| Urgent ablation in patients with ventricular dysfunction and an implanted ICD experiencing incessant VT or electrical storm causing ICD shocksb | I | B |
| Ablation of VE that triggers recurrent VF causing ICD shocks or electrical stormb | I | B |
| In patients with ischemic heart disease or ventricular dysfunction using an ICD that delivers recurrent shocks due to SVT | I | B |
| First-line treatment for patients with bundle branch reentrant SVT | I | C |
| As an alternative to ICD placement in patients with congenital heart disease and recurrent monomorphic SVT, or for patients with ICDs receiving appropriate therapy that cannot be controlled by reprogramming the device or medical treatment | I | C |
| In patients with ischemic heart disease or ventricular dysfunction using an ICD, following a first SVT episode | IIa | B |
| In patients with ARVC and SVT episodes refractory to medical treatment to prevent ICD shocksb | IIa | B |
| As an alternative to medical treatment for patients with congenital heart disease, ICD placement, and symptomatic monomorphic VT | IIa | B |
| For patients with dilated cardiomyopathy and VT refractory to medical treatment (except bundle branch reentrant VT) | IIb | C |
| For patients with symptomatic idiopathic VT or VE, or following treatment failure, or when there is suspected tachycardiomyopathy | IIb | B |
| Amiodarone | ||
| After a first SVT episode in patients with ventricular dysfunction and an ICD | IIa | B |
| In patients with an ICD delivering appropriate recurrent shocks despite optimal device programming | IIa | C |
| Patients with VF or VT and an indication for ICD implantation, when the device is not available, or is contraindicated because of concurrent medical reasons, or the patient refuses this treatment | IIb | C |
| Other arrhythmic drugs | ||
| Quinidine for patients with Brugada syndrome and an indication for ICD when they have a contraindication, refuse implantation, or require supraventricular arrhythmia treatment | IIa | C |
| Flecainide plus BB for patients with an implanted ICD to reduce appropriate shocks, and for patients with CPVT, PVT or recurrent bidirectional VT under BB treatment when ICD is not available, the patient has contraindications for the device, or refuses implantation | IIa | C |
| Quinidine or sotalol for patients with SQTS and an indication for ICD when they have a contraindication or refuse implantation | IIb | C |
| Surgical ablation | ||
| For patients with SVT refractory to antiarrhythmic drug therapy and catheter ablation performed by an expert electrophysiologistb | I | B |
| During cardiac surgery (coronary revascularization, valve surgery, or surgery for congenital heart disease) in patients with clinically documented SVT | IIb | C |
| Ventricular assist device | ||
| For hemodynamically unstable patients with recurrent SVT or VF despite optimal treatment | IIa | B |
| Left cardiac sympathetic denervation | ||
| For patients with symptomatic LQTS and an indication for ICD when they have contraindications, refuse implantation, or experience appropriate shocks while receiving BB treatment | IIa | C |
| For patients with CPVT and PVT or bidirectional VT, or those who have ICD shocks while receiving treatment with BB or BB + flecainide, or patients with intolerance or contraindications | IIb | C |
ARVC, arrhythmogenic right ventricular cardiomyopathy; BB, beta blockers; CPVT, catecholaminergic polymorphic ventricular tachycardia; ICD, implantable cardioverter-defibrillator; E, evidence level; I, indication; SCD, sudden cardiac death; SQTS, short QT syndrome; LQTS, long QT syndrome; PVT, polymorphic ventricular tachycardia; SVT, sustained ventricular tachycardia; VE, ventricular extrasystoles; VF, ventricular fibrillation; VT, ventricular tachycardia.
In the absence of reversible causes and not within the first 48 h following acute myocardial infarction, under optimal medical treatment, and with expected survival of >1 year in good functional status.
In specialized centers or those with related experience.
Based on data from the 2015 European Society of Cardiology guidelines on the management of patients with ventricular arrhythmias and sudden cardiac death prevention.23
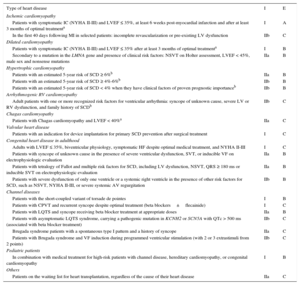
Indications for Implantable Cardioverter-defibrillator Placement for Primary Prevention
| Type of heart disease | I | E |
|---|---|---|
| Ischemic cardiomyopathy | ||
| Patients with symptomatic IC (NYHA II-III) and LVEF ≤ 35%, at least 6 weeks post-myocardial infarction and after at least 3 months of optimal treatmenta | I | A |
| In the first 40 days following MI in selected patients: incomplete revascularization or pre-existing LV dysfunction | IIb | C |
| Dilated cardiomyopathy | ||
| Patients with symptomatic IC (NYHA II-III) and LVEF ≤ 35% after at least 3 months of optimal treatmenta | I | B |
| Secondary to a mutation in the LMNA gene and presence of clinical risk factors: NSVT on Holter assessment, LVEF < 45%, male sex and nonsense mutations | IIa | B |
| Hypertrophic cardiomyopathy | ||
| Patients with an estimated 5-year risk of SCD ≥ 6%b | IIa | B |
| Patients with an estimated 5-year risk of SCD ≥ 4%-6%b | IIb | B |
| Patients with an estimated 5-year risk of SCD < 4% when they have clinical factors of proven prognostic importanceb | IIb | B |
| Arrhythmogenic RV cardiomyopathy | ||
| Adult patients with one or more recognized risk factors for ventricular arrhythmia: syncope of unknown cause, severe LV or RV dysfunction, and family history of SCDb | IIb | C |
| Chagas cardiomyopathy | ||
| Patients with Chagas cardiomyopathy and LVEF < 40%a | IIa | C |
| Valvular heart disease | ||
| Patients with an indication for device implantation for primary SCD prevention after surgical treatment | I | C |
| Congenital heart disease in adulthood | ||
| Adults with LVEF ≤ 35%, biventricular physiology, symptomatic HF despite optimal medical treatment, and NYHA II-III | I | C |
| Patients with syncope of unknown cause in the presence of severe ventricular dysfunction, SVT, or inducible VF on electrophysiologic evaluation | IIa | B |
| Patients with tetralogy of Fallot and multiple risk factors for SCD, including LV dysfunction, NSVT, QRS ≥ 180 ms or inducible SVT on electrophysiologic evaluation | IIa | B |
| Patients with severe dysfunction of only one ventricle or a systemic right ventricle in the presence of other risk factors for SCD, such as NSVT, NYHA II-III, or severe systemic AV regurgitation | IIb | B |
| Channel diseases | ||
| Patients with the short-coupled variant of torsade de pointes | I | B |
| Patients with CPVT and recurrent syncope despite optimal treatment (beta blockers±flecainide) | I | C |
| Patients with LQTS and syncope receiving beta blocker treatment at appropriate doses | IIa | B |
| Patients with asymptomatic LQTS syndrome, carrying a pathogenic mutation in KCNH2 or SCN5A with QTc > 500 ms (associated with beta blocker treatment) | IIb | C |
| Brugada syndrome patients with a spontaneous type I pattern and a history of syncope | IIa | C |
| Patients with Brugada syndrome and VF induction during programmed ventricular stimulation (with 2 or 3 extrastimuli from 2 points) | IIb | C |
| Pediatric patients | ||
| In combination with medical treatment for high-risk patients with channel disease, hereditary cardiomyopathy, or congenital cardiomyopathy | I | B |
| Others | ||
| Patients on the waiting list for heart transplantation, regardless of the cause of their heart disease | IIa | C |
AV, atrioventricular; CPVT, catecholaminergic polymorphic ventricular tachycardia; HF, heart failure; IC, ischemic cardiomyopathy; ICD, implantable cardioverter-defibrillator; E, evidence level; I, indication; LQTS, long QT syndrome; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSVT, non-sustained ventricular tachycardia; NYHA: New York Heart Association; PVT, polymorphic ventricular tachycardia; RV, right ventricular; SCD, sudden cardiac death; SQTS, short QT syndrome; STV, sustained ventricular tachycardia; VE, ventricular extrasystoles; VF, ventricular fibrillation; VT, ventricular tachycardia.
With expected survival of at least 1 year, following a detailed clinical evaluation that takes into account the lifelong risk of complications and the impact of implantable cardioverter-defibrillator on lifestyle, socioeconomic status, and psychological health, and indicates a net benefit from implantable cardioverter-defibrillator therapy.
Based on data from the 2015 European Society of Cardiology guidelines on the management of patients with ventricular arrhythmias and sudden cardiac death.23 These guidelines do not contemplate ICD implantation for patients with left ventricular ejection fraction < 30% in New York Heart Association functional class I and do not take into consideration indications such as those of Multicenter Automatic Defibrillator Implantation Trial and Multicenter Unsustained Tachycardia Trial.
Because of the difficulty of performing randomized studies showing an increase in survival with ablation of ventricular tachycardia (VT), indirect signs of the benefits of this treatment are being sought in the large registries. A meta-analysis involving 998 patients with ischemic heart disease and VT undergoing catheter ablation is one such effort. Noninducibility following ablation was associated with greater survival.24 This association may be limited by the left ventricular ejection fraction (LVEF) and be found only in patients with LVEF > 30%.25
Over the last few years the aim of this technique has been expanded toward more extensive ablation that will achieve noninducibility of any VT. Two recently published studies, mainly including patients with ischemic heart disease, have focused on this objective. The first used the scar dechanneling technique, which addresses the conducting channel exit and entrance points within the scar, followed by standard techniques of VT induction, mapping, and ablation, when needed.26 The second article reported a technique that directly addresses all the delayed potentials within the scar.27 Both studies included a sample of around 100 patients and reported good results at 20 months, with VT recurrence rates of 28% and 32%, respectively.
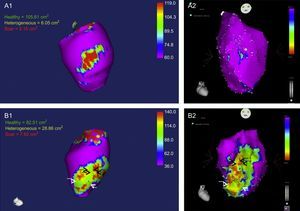
Continuing with the objective of extensive ablation, systematic use of a combined endo-epicardial approach in unselected ischemic patients was related to a lower rate of hospitalizations for VT and repeat ablation compared with endocardial ablation alone.28 However, a small percentage of patients have no scar or no treatable substrate in the epicardium.28 An experimental study in pigs with chronic infarction published last year29 showed the potential value of cardiac magnetic resonance imaging to identify patients with these features. The study reported that detection of “heterogeneous gray areas” in the most external epicardial layer using gadolinium perfusion predicted VT inducibility originating in the epicardium on electrophysiologic evaluation (Figure 1).29
A1 and A2: Images obtained in a pig without inducible ventricular tachycardia, showing a small epicardial scar with minimal extension of heterogeneous tissue (A1, magnetic resonance; A2, CARTO voltage map). B1 and B2: Images obtained in a pig with inducible epicardial ventricular tachycardia, depicting an epicardial scar with a patchy pattern and at least 3 areas of dense scar surrounded by heterogeneous tissue. Arrows indicate channels. Images courtesy of Drs. Angel Arenal and Ester Pérez David.
As to ventricular outflow tract arrhythmias, a pulmonary valve distance of > 1cm from the earliest electrogram recorded in the right ventricular outflow tract was found to predict a left ventricular origin.30 Another study has confirmed the difficulty of ablation treatment for this type of arrhythmia when the origin is located close to the area known as the ventricular “summit”, reporting an acute efficacy of around 50%.31
Implantable Cardioverter-defibrillatorData from the Spanish Implantable Cardioverter Defibrillator Registry for 201332 showed an increase in the number of devices implanted relative to the previous year (4772 vs 4216), exceeding the number recorded in 2010, which had been the highest (4627 implants) since the start of the Registry. According to the EUCOMED (European Confederation of Medical Suppliers Associations) data, the implant rate per million population in Spain has remained at the low end of rates in other European countries and shows the largest difference compared with the mean value (120 vs a mean of 289 in Europe).
The current technological advances and accumulated experience cast doubt on the need for a defibrillation test during device implantation. SIMPLE33 is a multicenter noninferiority study performed in 2500 patients with an indication for ICD implantation. The patients were randomized at 1:1 to undergo a defibrillation threshold test or not. After a mean follow-up of 3.1 years, there were no differences between the groups regarding the composite endpoint of failed appropriate shock or arrhythmic death, providing robust evidence of the safety of omitting this test.
Defibrillation threshold testing is still being used in subcutaneous ICD implantation, for which there is less experience. Arias et al34 reported the largest experience in Spain with this therapy, describing favorable outcomes. Combined analysis of the data from 852 patients with subcutaneous ICDs35 showed excellent effectiveness, with no cases of electrode failure and a 5% rate of inappropriate shocks in the last quartile of patients included.
A subanalysis of the ADVANCE III36 study has shown that programming long detection times in transvenous ICDs avoids treatment of self-limiting arrhythmia even in patients undergoing secondary prevention, with no increase in syncopal episodes.
Risk Stratification for Sudden Death in Patients with Structural Heart DiseaseA new SCD risk prediction model has been developed and validated37 in hypertrophic myopathy. This model estimates individual 5-year risk by using clinical variables and is of help in decision-making on treatment such as ICD implantation. Furthermore, it improves the low positive predictive value for SCD of previous models, which was clearly evident in the study by Sarrias et al.38
Identification of patients who can best benefit from an ICD is a complex problem that remains unresolved by analyzing the profile of those receiving this treatment. In this regard, measurement of serum biomarkers may help to identify patients at a high risk of death before they have ventricular arrhythmias, as was seen in the PROSE-ICD39 study.
It is also important to identify the predictors of morbidity and mortality following ICD replacement, particularly in elderly patients and those with severe comorbidities, in whom the risks of replacement may outweigh the expected clinical benefit. The REPLACE DARE40 risk score was developed to identify patients with a high or low risk of death at 6 months following replacement of implantable electronic cardiac devices, including ICDs.
Channel DiseasesThe FIVI-Gen41 study is a multicenter Spanish registry of patients with idiopathic ventricular fibrillation undergoing a sequential diagnostic algorithm including pharmacologic tests, familial evaluation, and genetic assessment. This is the first study to report the value of large-scale genetic testing in the absence of the phenotype in probands, and the diagnostic usefulness of assessing family members.41
In the treatment of long QT syndrome, a study analyzing the utility of different beta-blockers determined according to the underlying genotype found that propranolol may be less effective in patients with previous clinical events. Nadolol would be preferentially used in type 2 long QT syndrome, but no significant differences between the 4 beta-blockers analyzed were found in Type 1.42
Knowledge of the long-term effects of cardiac sympathectomy has led to advances in the therapeutic strategy for catecholaminergic polymorphic VT. De Ferrari et al43 reported the longest mean follow-up to date (37 months) in a study including 63 patients with this channel disease undergoing cardiac sympathectomy with or without optimal medical therapy. The data indicated a beneficial long-term effect, with a significant reduction in the rates of syncope, appropriate ICD shocks, and SCD in patients treated for secondary prevention.
Controversy persists regarding the value of programmed ventricular stimulation in arrhythmic risk stratification to predict SCD risk in patients with Brugada syndrome. Once again, data from a study with a lengthy follow-up suggest that ventricular fibrillation inducibility with programmed stimulation is predictive of SCD.44
ADVANCES IN THE MANAGEMENT OF PATIENTS WITH SYNCOPEImportance of Syncope UnitsThere is increasingly more evidence indicating the need to protocolize the management of patients with syncope in specialized units, as this measure leads to lower morbidity and mortality, a higher diagnostic yield, and reductions in cost. In this line, the European Heart Rhythm Association (EHRA) published a consensus document proposing a systematic approach for the care of syncope patients in dedicated units.45
Underuse of Insertable Cardiac MonitorsDespite the current clinical evidence supporting an essential role for insertable event recorders in the management of recurrent syncope of unknown origin, a retrospective survey by the EHRA has shown that this resource is underused in Europe relative to the recommended use in clinical practice guidelines.46
Prognostic Aspects: Predictors of Mortality and the Need for Pacemaker ImplantationBrignole et al47 reported that proven asystole during carotid sinus massage, tilt table testing, or insertable cardiac monitoring in patients older than 40 years predicts a future benefit from pacemaker implantation. Along the same line, a study carried out in patients with syncope or unexplained presyncope using an insertable event recorder showed that a history of trauma attributable to syncope is the leading independent predictor of pacemaker implantation.48
In a study in syncope patients, Pérez-Rodón et al49 reported that several electrocardiographic parameters, including AF, intraventricular conduction disturbances, ventricular hypertrophy, and ventricular pacing rhythm had independent prognostic value for mortality.
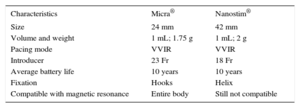
DEVELOPMENTS IN CARDIAC PACINGLeadless PacemakersThere are currently 2 models of single-chamber devices (Nanostim® from St. Jude Medical® and Micra® from Medtronic®), equipped with release systems and directly attached to the apical or basal septal region of the right ventricular wall using a percutaneous delivery sheath introduced through the femoral vein (Figure 2 and Table 3).50
Characteristics of Leadless Pacemakers
| Characteristics | Micra® | Nanostim® |
|---|---|---|
| Size | 24 mm | 42 mm |
| Volume and weight | 1 mL; 1.75 g | 1 mL; 2 g |
| Pacing mode | VVIR | VVIR |
| Introducer | 23 Fr | 18 Fr |
| Average battery life | 10 years | 10 years |
| Fixation | Hooks | Helix |
| Compatible with magnetic resonance | Entire body | Still not compatible |
Ritter et al51 presented the results of a prospective, uncontrolled, multicenter study on the safety and efficacy of the Micra® device, including 140 patients. The implantation success rate was 100%, with a mean duration of the procedure of 37±21 minutes and fluoroscopy time of 9±7 minutes. No severe adverse events occurred over a mean follow-up of 1.9±1.8 months.
Anticoagulation Management in Patients With Implanted DevicesA new meta-analysis has reinforced the evidence that continuing with oral anticoagulation is safe compared with heparin bridging therapy in patients requiring implantation or replacement of a cardiac pacing device. The results show a reduction in the incidence of bleeding events, with no differences in thromboembolic risk.52
Cano et al53 evaluated the strategy of continuing with oral anticoagulation irrespective of the individual thromboembolic risk in 278 patients with an INR (international normalized ratio) of 2 to 4 undergoing implantation of a first device or device replacement. There was a low incidence of pocket hematoma (2.9%) and there were no thromboembolic events.
Remote Pacemaker MonitoringMost studies published in the past year have focused on the reduction in morbidity and mortality associated with remote monitoring of implantable cardiac devices. The IN TIME54 clinical trial showed improvements in the symptoms and prognosis of patients with a cardiac resynchronization therapy defibrillator (CRT-D) alone undergoing RM. After a 1-year follow-up, there was a significant decrease in the primary endpoint, consisting of a clinical score that combined (among other variables) all-cause death, hospitalization for heart failure, and functional class change. These results were supported by those of an observational study published by Varma et al55 including a cohort of 269 471 patients with a pacemaker, ICD, or cardiac resynchronization therapy (CRT) with pacing or defibrillation capability.
CARDIAC RESYNCHRONIZATION THERAPYNew Clinical Practice GuidelinesThe new European Society of Cardiology guidelines for the management of patients with ventricular arrhythmias and the prevention of SCD have updated the indication for CRT (Figure 3).23
Indications for cardiac resynchronization therapy ± implantable cardioverter-defibrillator according to the European Society of Cardiology guidelines.23 AF, atrial fibrillation; AVN, atrioventricular node; BiV, biventricular; CRT, cardiac resynchronization therapy; LBBB: left bundle branch block; FC, functional class; LVEF, left ventricular ejection fraction; HF, heart failure; ICD, implantable cardioverter defibrillator; NYHA: New York Heart Association. aIn good functional status. bProvided that biventricular pacing close to 100% is anticipated. cIrrespective of QRS morphology and to reduce hospitalization for heart failure.
The use of left ventricular quadripolar leads in CRT is associated with a reduction in the procedure and fluoroscopy times and with a lower incidence of phrenic nerve stimulations and lead displacements. Recently, Behar et al56 demonstrated their beneficial effect on overall mortality.
Two studies have reported more favorable results with multipoint left ventricular pacing than with conventional biventricular pacing regarding acute hemodynamic parameters (cardiac output and LVEF)57 and left ventricular remodeling parameters (LVEF and end-systolic volume).58
Predictors of Response to Cardiac Resynchronization TherapyThe results of a meta-analysis have confirmed the deleterious effect of CRT in patients with a QRS complex duration < 130 ms, showing an increase in mortality that was significant even in patients with asynchrony on echocardiography.59
Another meta-analysis, in this case including more than 4000 patients in New York Heart Association functional class II randomized to ICD or CRT-D, demonstrated a greater decrease in mortality and heart failure in patients with CRT-D. This benefit was mainly seen in patients with left bundle branch block, in women with QRS ≥ 130 ms, and in men with QRS ≥150 ms.60
The presence of atrial and ventricular extrasystoles has been associated with a suboptimal percentage of biventricular pacing. In a recent study, ≥ 0.1% of extrasystoles was related to poorer ventricular remodeling, increased total mortality, and the development of heart failure.61
There is also some new information on the electrode activation time as a predictor of response to CRT. De Riva-Silva et al62 observed that an activation time of ≥ 72 ms can differentiate between responders and nonresponders with a sensitivity of 83% and specificity of 71%.
Ventricular Arrhythmia in Patients with Cardiac Resynchronization TherapyTwo studies have shown that even though the incidence of appropriate therapy is lower in responders, it still occurs even in super-responders. Over a mean follow-up of 30 months, García-Lunar et al63 observed that the risk of appropriate therapy was 24.2% in nonresponders, 22.2% in responders, and 5.9% in super-responders. In a substudy of the MADIT-CRT trial, the results were similar: the risk of appropriate therapy over a mean follow-up of 3 years following CRT was 6% in patients with LVEF > 50%.64
CONFLICTS OF INTERESTNone declared.