Keywords
INTRODUCTION
Smoking causes 11% of all mortality cardiovascular,1 while recent studies in Spain indicate that the incidence of cardiovascular disease attributable to smoking in men is approximately 40%.2 Tobacco consumption independently increases the risk of coronary, cerebral, and peripheral atherosclerotic disease,3 both in active and passive smokers.4 However, the pathogenesis of cardiovascular disease associated with smoking is not completely understood, though it has been linked with endothelial dysfunction, alterations in the balance between coagulation and fibrinolysis, and metabolic lipid changes,5,6 areas in which vascular inflammation and oxidative stress would play a central role.7
Recent studies indicate that an alteration in the balance between synthesis and degradation of the extracellular matrix (ECM) could also contribute to the development of cardiovascular disease associated with smoking.8 The metalloproteinases (MMP), a family of endopeptidases that break down various components of the ECM, are classified into subgroups based on their structure, substrate specificity, and membrane binding properties: collagenase (MMP-1, MMP-8, MMP-13), stromelysin (MMP-3, MMP-10, MMP-11), gelatinase (MMP-2, MMP-9), membrane type (MTMMP), and others (matrilysin, metaloelastase, etc). Intra- and extracellular MMP activity is regulated through transcription, postranslation, and through interaction with specific inhibitors (TIMP).9
The MMPs have been implicated in the inflammatory, angiogenic, and proteolytic processes that accompany angiogenic remodeling and the break-up or erosion of vulnerable plaque which is also associated with smoking.8 An increase in the number of MMPs has also been linked to cardiovascular disease, which has led to calls for them to be used as a biomarkers.9-11 Their importance in the development of chronic obstructive pulmonary disease (COPD), the second leading cause of death associated with smoking, is also well-known.12,13
Recent studies by our group have shown a strong link between inflammation, subclinical atherosclerosis, and increases in circulating MMP-10 (stromelysin 2),14,15 an MMP that breaks down type III and IV collagen, elastin, and proteoglucanes, and which is also present at increased levels in atherosclerotic plaques in patients with advanced atherosclerosis.14
The objective of this study was to determine whether MMP-10 was associated with smoking in asymptomatic subjects, independently of other risk factors. If that was the case, it could be relevant to the atherogenic process through its effects on ECM. To answer the study question, we quantified the levels of several circulating MMPs and TIMP-1 in both smokers and non-smokers without cardiovascular disease.
METHODS
We analyzed a group of 400 apparently healthy subjects (mean age [range], 54.3 [20-80] years). Subjects were predominantly male and have been described previously.15 Samples were taken sequentially during the period 2003-2005 when subjects attended the outpatient service of the Department of Internal Medicine at the University Clinic of Navarra for a general checkup. Subjects were selected by the attending physician from a population of 414 subjects without evidence of symptomatic cardiovascular disease according to the following criteria: a) no history of heart disease, stroke, or peripheral arterial disease; and b) normal electrocardiogram and chest x-ray. Coronary artery disease was defined as: a) a history of acute myocardial infarction (AMI), angina, or use of nitroglycerine; and b) a history of coronary angioplasty or bypass surgery. Cerebrovascular disease was defined as a history of stroke, transient ischemic attack, or carotid endarterectomy. Any symptoms of intermittent claudication were recorded and an exploration was performed to evaluate the peripheral pulses. Other exclusion criteria were: substantial alteration of renal function (glomerular filtration <60 mL/min), presence of chronic inflammatory disease, and administration of anti-inflammatory medication, antithrombotic or hormone therapy in the 2 previous weeks. Subjects with acute infection based on clinical criteria were also excluded.
Assessment of Smoking and Other Cardiovascular Risk Factors
We used a standardized questionnaire on tobacco consumption to classify subjects as smokers (at least 1 cigarette daily on average over the past year), never smokers, and former smokers (no cigarette consumption in the past year).
In addition, we obtained information about other atherosclerotic risk factors, including diabetes mellitus, high blood pressure, dyslipidemia, and obesity. Subjects were considered hypertensive if they had a systolic blood pressure (SBP) >139 mm Hg and / or diastolic blood pressure (DBP) >89 mm Hg, and / or if they were taking antihypertensive drugs. Dyslipidemia was diagnosed when total cholesterol values were ≥200 mg/dL, LDL cholesterol (LDL-C) was ≥130 mg/dL, HDL cholesterol (HDL-C) was ≤50 mg/dL, triglycerides were ≥150 mg/dL and / or if the subject was taking lipid-lowering medication. Subjects were classified as obese if they had a body mass index (BMI) of ≥30 and diabetes mellitus was considered present when glucose values were > 126 mg/dL or if the subject was taking anti-diabetic medication.
We calculated total vascular risk using the REGICOR charts,16 which have been validated in the Spanish population, and PROCAM,17 which was validated in the German population.
Informed consent was obtained from all subjects and the study was approved by the Institutional Review Board in accordance with the Declaration of Helsinki (October 2000 revision).
Procedures
Fasting plasma and serum samples were extracted between 8:00 am and 10:00 am by venipuncture. They were centrifuged (20 min, 1200 g) and frozen at -80° C until analysis.
Metabolic Profile
We measured total cholesterol, HDL-C, triglycerides, and glucose using standard laboratory techniques in fasting blood samples. Levels of LDL-C were calculated using the Friedwald equation.
Inflammatory Markers
Plasma fibrinogen activity was measured using Clauss' method. Concentrations of high sensitivy-C-reactive protein (hs-CRP) (IMMULITE Diagnostic Product Corporation, USA), interleukin (IL) 6 (Quantikine R & D Systems, UK ) and von Willebrand factor (VWF) (Asserachrom Diagnostica Stago, France) were determined using ELISA following the manufacturer's instructions. Inter- and intra-analysis coefficients of variation for ELISA were under 6%.
Proteolytic Markers
Plasma concentrations of MMP-1, MMP-9, and TIMP-1, and serum concentrations of MMP-10 were estimated using ELISA (R & D systems, USA) following the manufacturer's instructions. Inter- and intra-analysis coefficients of variation for ELISA were under 6%.
Determining Carotid Intima Media Thickness (IMT)
Carotid intima media thickness (IMT) was measured in all subjects by Doppler ultrasound of the common carotid arteries (CCA), as described previously.15 A 5-12 MHz linear transducer (ATL HDI 5000) was used. The IMT was measured in regions without plaque 1 cm from the carotid bulb in each CCA. Two echocardiograph technicians who were unaware of the clinical history determined mean readings for each CCA.
Statistical Analysis
Quantitative variables are expressed as mean (standard deviation). The Kolmogorov-Smirnov test was used to test the normality of the distributions. Log transformations were used to normalize hs-CRP values for further statistical analysis. Differences in mean values and proportions in relation to sex and smoking were analyzed using Student t test and c2, respectively. Former smokers were excluded from the analysis.
Pearson's correlation coefficient was used to analyze correlations between concentrations of inflammatory and proteolytic markers, and cardiovascular risk factors. We calculated the marginal means for smokers and non-smokers using analysis of variance (ANCOVA), after adjusting for age, sex, and cardiovascular risk factors. A multivariable linear regression analysis was used to determine whether the association between MMP-10 and smoking was independent of these risk factors and to estimate the relative importance of each variable. All variables were entered using stepwise procedures. The statistical analysis was performed with SPSS 11.0. A P value less than .05 was considered significant.
RESULTS
A non-randomized sample of 400 subjects without clinical cardiovascular disease was included (mean [interval] age, 54.3 [20-80] years; 78% male). There were high proportions of subjects with dyslipidemia (81%), high blood pressure (50.7%), and obesity (32%), and a lower prevalence of subjects with diabetes (16%). The study population's clinical characteristics and cardiovascular risk factors are shown in Table 1 for the overall sample and by gender. Male subjects (n=311) were younger (P<.05), with a higher proportion of smokers and former smokers (P<.01). They also had significantly lower levels of HDL-C and higher triglyceride and glucose values (P<.01) than female subjects (n=89). Total vascular risk on the REGICOR chart (P<.01) and IMT values (P<.01) were higher in men.
Table 2 shows the differences in cardiovascular risk factors, inflammatory and proteolytic markers among the group of active smokers (n=118) and never-smokers (n=195). Smokers were younger and predominantly male (P<.001). While no difference was seen between groups in the prevalence of other cardiovascular risk factors or fibrinogen levels, PCR-us, VWF, IL-6, MMP-9, or TIMP-1, MMP-1 (P<0.05), and MMP-10 values were significantly higher (P<.001) in smokers. No significant differences were seen for IMT.
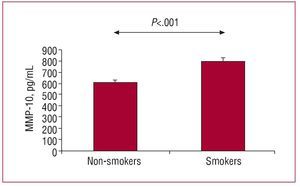
The results of analyzing correlations for both MMPs are shown in Table 3. MMP-1 was correlated with LDL-C (P<.01), total cholesterol, and TIMP-1 (P<.05), whereas MMP-10 showed a significant correlation with age (P<.05), HDL-C (P<.05), fibrinogen (P<.01), IMT (P<.05), and TIMP-1 (P<.01), as well as with PROCAM and REGICOR scores (P<.01). After adjusting for age, sex, and cardiovascular risk factors which were significant in the univariate analysis, only MMP-10 was still statistically significantly higher (P<.001) in smokers (Figure 1). Finally, a multiple regression analysis, in which standardized regression coefficients were defined, was performed to assess the relative importance of each of the variables which were independently associated with MMP-10 concentrations. As shown in Table 4, the variables that contributed significantly to the variations in MMP levels were age (15%, P<.01) and, to a greater extent, smoking (28%, P<.001).
Figure 1. Concentrations of MMP-10 in smokers and non-smokers without cardiovascular disease. Analysis of covariance (ANCOVA) adjusted for cardiovascular risk factors which were significant in the univariate analysis
DISCUSSION
In a series of subjects without cardiovascular symptoms, concentrations of circulating MMP-10 (stromelysin 2) were significantly higher in smokers compared to non-smokers. The association between MMP-10 and smoking was independent of other, traditional risk factors. In fact, smoking made the greatest contribution in explaining variability in levels of MMP-10. These results suggest that high levels of MMP-10 may be an additional cardiovascular risk factor associated with smoking in subjects without symptomatic cardiovascular disease. Of added interest is the fact that this molecule mediates the degradation of ECM, the erosion and breakdown of which are related to atherothrombotic syndromes.8,9 A correlation between MMP-10 and total cardiovascular risk, estimated using the PROCAM and REGICOR scores, was also observed. Nevertheless, the correlation was weak making its clinical significance uncertain. Finally, MMP-10 correlated with IMT, a marker of subclinical atherosclerosis.15 In the multivariate analysis, age (15%) and smoking (28%) made the greatest contribution to high levels of circulating MMP-10.
We also found a correlation between MMP-1 (collagenase 1) and smoking, which confirmed previous survey data.18-21 No correlation with other MMPs or TIMP-1 was observed. Overall, the results of this study suggest that an alteration in the balance between synthesis and degradation of ECM can, by increasing the concentrations of several MMPs, play an important part in the genesis of cardiovascular changes associated with tobacco consumption in subjects asymptomatic for cardiovascular disease.8
Smoking is an independent risk factor for coronary, cerebrovascular, and peripheral atherosclerosis, as well as for aortic aneurysms.22,23 The simultaneous occurrence of factors, such as cholesterol or high blood pressure, does not adequately explain the increased cardiovascular risk associated with smoking.24,25 However, a synergy between various MMPs and smoking in relation to cardiovascular disease has been observed.26,27 An excessive inflammatory and oxidative response, mediated by cytokines and NF-kB or AP-1 type transcription factors, appears to be important in the over-expression of MMPs in smokers.8,28-32 It is also possible, given the close relationship between smoking and COPD, that the higher levels of MMP-10 originate in the lungs.13
In our study, MMP-10 was found to have a particularly strong association with tobacco consumption. MMP-10 is released by the endothelium in response to inflammatory stimuli, which can be significantly involved in vascular remodeling and the appearance of atherothrombotic complications.9,33 Recent studies by our group have shown higher levels in asymptomatic subjects with cardiovascular risk factors and subclinical atherosclerosis,15 as well as in patients with established atherosclerotic disease.14 Interestingly, MMP-10 has also been associated with aortic aneurysms.34 The results of this study emphasize the importance of this MMP in smokers, whereas other markers of inflammation and endothelial damage such as fibrinogen, IL-6, and VWF associated with subclinical atherosclerosis in asymptomatic subjects,35,36 appear to be less useful in assessing smoking-related cardiovascular risk. While MMP-10 was correlated with age in the study population, it is interesting to note that MMP-10 levels were elevated in smokers, who were generally younger. These results were confirmed in multivariate analysis after adjusting for this variable.
Various mechanisms have been proposed to explain the induction of MMP through smoking, both in vitro and in vivo.6,37,38 The exposure of endothelial cells in vitro to tobacco smoke induces the expression of MMP-1, MMP-8, and MMP-919,32; in a similar fashion, tobacco smoke stimulates the production of MMP-1 through human fibroblasts.21 While increased levels of cadmium inhaled with tobacco smoke could induce proteinolysis, as demonstrated in COPD,13 they could also contribute to cardiovascular disease, as suggested by increased levels in the aorta of smokers39 and in patients with peripheral arterial disease.40
Unlike other studies in patients with clinical cardiovascular disease,26,27,29,38 we did not find any differences between smokers and non-smokers in terms of other MMPs, such as MMP-9 or TIMP-1; this may be related to the type of sample analyzed and the exclusion of patients with clinical atherosclerosis.
The study has several limitations. The cross-sectional design means it is not possible to establish causal associations between proteolytic marker values and cardiovascular risk; prospective studies are required for that. The low number of women included and the fact that the sample was not randomized means the results can not be extrapolated to the general population. Finally, some MMP values are subject to circadian variations that cannot be detected in a cross-sectional sample, although all extractions were performed in the same time period.41
CONCLUSIONS
The group of asymptomatic smokers studied here showed an increase in MMP-10 that was independent of other traditional atherosclerotic risk factors. The close relationship between tobacco consumption and MMP-10 and between the latter and the regulation of EMC could indicate that this MMP plays a key role in the atherosclerotic process associated with smoking.
ABBREVIATIONS
IMT: carotid intima media thickness
ECM: extracellular matrix
IL-6: interleukin 6
MMP: metalloproteinase
MT-MMP: membrane type metalloproteinase TIMP: tissue inhibitor of metalloproteinases
Financed by UTE project CIMA (Universidad de Navarra, Pamplona, Navarra, Spain) and Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (RECAVA RD06/0014/0008).
Correspondence: Dr. J.A. Páramo.
Laboratorio de Aterosclerosis. Área de Ciencias Cardiovasculares, CIMA. Avda. Pío XII, 55. 31008 Pamplona. Navarra. España.
E-mail: japaramo@unav.es
Received February 20, 2008.
Accepted for publication August 29, 2008.