Keywords
INTRODUCTION
Atherosclerotic cardiovascular disease is a growing global public health challenge. Despite the advances in the treatment of clinically manifest coronary artery disease, recurrences are common. Most importantly, initiation of treatment after a clinical event is usually a therapy too late. Accordingly, the search continues to identify strategies that effectively reduce vascular risk. Demonstration of a reduction of clinical events, in response to particular therapies, remains the sine qua non of proof of benefit. However, assessing the impact of potential preventive strategies must be performed on the background of a combination of multiple agents with proven efficacy. High rates of concomitant administration of aspirin, beta-blockers, and statins, in addition to aggressive control of blood pressure and glucose, will result in a substantial lowering of event rates in placebo arms. As a result, it will become necessary to enrol increasingly large numbers of patients, followed for many years to achieve an adequate event rate in order to assess the therapeutic efficacy of experimental agents. The emergence of imaging modalities that characterize the arterial wall provide a unique opportunity to monitor changes in the size and morphology of atherosclerotic plaque, in response to the administration of potential anti-atherosclerotic therapies. Studies using such an imaging modality may provide valuable preliminary information for the design of much larger randomised controlled trials.
EFFECT OF INTERVENTIONS ON ESTABLISHED ATHEROSCLEROSIS
A number of lipid lowering agents reduce the incidence of clinical events in studies of both primary and secondary prevention.1-9 The precise mechanism that confers this benefit remains uncertain. Various groups have attempted to determine whether the reduction in clinical events is due to a reduction in atherosclerotic burden. Demonstrating plaque regression, in response to an intervention, has remained relatively elusive. This has stimulated considerable interest in the concept that the clinical benefit related to the use of these interventions is due largely to resulting changes in plaque composition rather than size. While this may be the case, there is currently no established method of assessing the changes in plaque characteristics in humans.
Coronary angiography is the clinical gold standard for the detection of significant luminal stenoses. It plays a pivotal role in clinical decision making, with regard to the implementation of a range of revascularization strategies. However, its ability to predict ischemic events is limited. Several groups have reported that the majority of myocardial infarctions occur in a vascular territory supplied by a culprit lesion, deemed to be only mildly stenotic at the time of angiography.10-12 However, it has become increasingly recognized that while coronary angiography is a valuable technique for imaging of the luminal border, it's ability to identify the total extent of atherosclerosis is suboptimal.13 This has led to the development of modalities that are more able to accurately image atherosclerotic plaque. Intravascular ultrasound (IVUS) has emerged as a technique that provides a unique opportunity to characterize the response of the arterial wall to serial changes in the accumulation of atherosclerotic plaque.
INTRAVASCULAR ULTRASOUND
IVUS involves the insertion of a catheter, whose tip contains a high frequency ultrasound transducer, within the epicardial coronary arteries. It can be safely performed at the time of diagnostic coronary angiography. IVUS generates high resolution tomographic cross-sectional images of the arterial wall, plaque and lumen. IVUS can be used in the interventional setting to guide decision making, with regard to the management of specific lesions. In addition, continuous imaging during withdrawal of the catheter generates a series of tomographic images that allow for a systematic assessment of atheroma burden. Pullbacks are performed manually or by connecting the catheter to a motorized device, which operates at a constant rate, typically 0.5 mm/s.
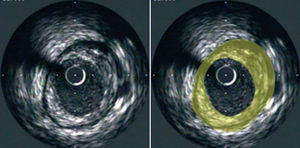
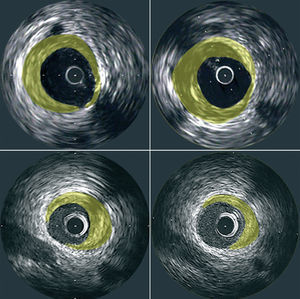
The resulting images provide information regarding the extent and composition of atherosclerotic plaque. Using planimetry it is possible to determine the area occupied by the external elastic lamina (EEM) and the lumen. Plaque area can then be calculated as the difference between EEM and lumen areas14 (Figure 1). With the generation of a series of consecutive images, resulting from the pullback of the catheter, the plaque areas from these tomographic images can be summated to estimate the volumetric burden of atheroma in the segment of artery studied. Most groups have typically measured images spaced exactly 1-mm apart for volumetric analysis. IVUS also provides an assessment of the presence, extent and location of calcification. The ability to perform IVUS studies within the same arterial segment at different time points highlights its potential to assess the natural progression of the atherosclerotic process and the impact of various anti-atherosclerotic interventions (Figure 2).
Figure 1. Cross-sectional tomographic image of a coronary artery obtained at IVUS pullback (left panel). Plaque area (yellow) is depicted as the area between the leading edges of the EEM and lumen (right panel).
Figure 2. Representative cross-sectional IVUS images demonstrating plaque progression (top panels) and regression (bottom panels) at matched sites that were studied at baseline (left panels) and follow-up (right panels).
IVUS HIGHLIGHTS THE NATURAL HISTORY OF ATHEROGENESIS
IVUS has provided a number of important insights into the response of the arterial wall to the deposition of atherosclerotic plaque. The ability to image the entire vascular wall allows for an accurate determination of plaque burden. IVUS has confirmed that atherosclerosis is a much more diffuse process than would be suggested by the examination of coronary angiograms.15 In addition, IVUS studies have demonstrated that this chronic process begins early in life. In a study of 262 patients that underwent cardiac transplantation, IVUS examinations were performed of the donor coronary arteries shortly following the transplant procedure. Significant atheroma, defined by a mean maximal plaque thickness greater than 0.5 mm, was found in 1 in 6 apparently healthy hearts from teenage donors. The prevalence of significant atheroma increased dramatically with age.16 These results confirm previous reports of the early appearance of macroscopic atheroma in necropsy studies17,18 and population studies using carotid intimal medial thickness as a surrogate measure of coronary atherosclerosis.19,20
It has become apparent that the vessel wall is not a passive player in the atherosclerotic process. Rather, the wall undergoes substantial changes in size and structure, in response to plaque accumulation. On the basis of necropsy studies, Glagov and colleagues described arterial remodeling as expansion of the internal elastic lamina, in response to the presence of atheroma.21 These changes, subsequently confirmed by IVUS studies,22,23 initially preserve lumen dimensions. It is therefore possible for the arterial wall to harbor a substantial amount of atheroma before it causes any detectable obstruction on angiography. Further studies have demonstrated that the direction of arterial remodeling correlates with the mode of clinical presentation. Culprit lesions in the setting of acute coronary syndromes are more likely to be associated with expansive remodeling, while constrictive remodeling correlates with stable angina pectoris.24
IVUS has identified the presence of multiple ruptured plaques within the coronary arteries in patients with a history of acute coronary syndrome.25,26 Plaque rupture has been found to be present, not only in the culprit vessel, but throughout the coronary arterial tree. This supports the concept that atherosclerosis and it's ischemic complications are part of a systemic and not focal process.27,28 It also confirms that the overwhelming majority of episodes of plaque rupture are not accompanied by clinical ischemia. This suggests that a combination of factors influence whether breakdown of the fibrous cap results in thrombosis and luminal occlusion.29
IVUS has also provided important insights into the natural history of nonatherosclerotic processes, such as transplant vasculopathy. IVUS is a sensitive approach for the detection of early neointimal thickening.30,31 Using serial assessments, IVUS has documented the incidence and subsequent rate of progression of these changes and the impact of interventions.32,33 As a result, serial IVUS imaging has become the clinical gold standard for the routine surveillance of the coronary arteries in heart transplant recipients.34
USE OF IVUS TO ASSESS THE IMPACT OF INTERVENTIONS ON THE NATURAL HISTORY OF PROGRESSION
The ability of IVUS to be performed on a serial basis and to quantify the extent of atherosclerotic plaque or neointimal thickening provides an exciting opportunity to assess the impact of therapeutic interventions. Several reports have emerged that describe the impact of strategies that modify plasma lipids, blood pressure, and the immunological mediators of transplant vasculopathy.
IMPACT OF HDL ELEVATION ON PLAQUE BURDEN
Despite the overwhelming evidence supporting a protective role of high density lipoproteins (HDL) in population35 and animal36-39 studies, data demonstrating a benefit from promoting HDL in humans is limited. It was recently reported that infusing reconstituted HDL particles containing apolipoprotein A-I Milano, a variant of apo A-I, promoted the rapid progression of coronary atheroma in humans.40 Forty seven patients, who underwent coronary angiography and IVUS within 2 weeks of an acute coronary syndrome were randomised to receive weekly intravenous infusions of either placebo or recombinant apo A-I Milano/phospholipid complexes (ETC-216) at a protein dose of 15 mg/kg or 45 mg/kg for 5 weeks. A repeat IVUS study was performed within 2 weeks of the final infusion. The combined treatment group, representing patients that received either low or high dose ETC-216, demonstrated a 4.2% reduction in atheroma volume. The degree of regression was greatest in the most severely diseased 10-mm subsegments. This result extends the findings that carriers of the apo A-I Milano variant appear to be relatively protected from coronary heart disease, despite low HDL levels,41 and that similar infusions in animal models exert a beneficial influence on lesion size and composition.42-44 Further, it extends the findings, that the combination of niacin and a statin, with resulting HDL elevation, promoted atherosclerotic regression in angiographic studies45 and halted progression of carotid intimal medial thickness.46 This small study is an important proof of concept that interventions that promote HDL have the potential to have a dramatic impact on coronary atheroma. It calls for the design of large scale studies to further investigate the potential impact that promoting HDL has on coronary atheroma and the incidence of clinical events.
IMPACT OF LIPID LOWERING ON PLAQUE BURDEN
It is well established that LDL lowering with statins reduces clinical events.1-6 However, it remains uncertain whether a LDL threshold exists, below which incremental clinical benefit is limited. This has stimulated considerable debate in the development of lipid lowering guidelines. Serial IVUS studies have documented the response of coronary atheroma to intensive lipid lowering strategies. Early reports of atherosclerotic regression were reported, using both quantitative coronary angiography and IVUS, following LDL apheresis in patients with familial hypercholesterolemia that was refractory to conventional medical therapy.47,48 Numerous angiographic studies have demonstrated that lipid lowering with statins slowed the rate of progression of atheroma in comparison with placebo.49-53 Recent IVUS studies have directly addressed the issue whether the degree of LDL lowering with statin therapy influences coronary atheroma.
The Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) study54 randomised 502 patients with coronary artery disease and an LDL-C level between 125 mg/dL and 210 mg/dL to receive either a moderate lipid lowering strategy with pravastatin 40 mg daily or an intensive lipid lowering strategy with atorvastatin 80 mg daily for 18 months. Mean LDL-C was reduced to 79 mg/dL and 110 mg/dL with atorvastatin and pravastatin respectively. In addition the strategies differed markedly in their ability to lower CRP. CRP decreased by 36.4% and 5.2% in atorvastatin and pravastatin treated patients respectively. Serial IVUS demonstrated that atheroma volume increased by 2.7% with pravastatin, indicating net progression. In contrast, treatment with atorvastatin resulted in no significant change of atheroma volume compared with baseline. Further, it was demonstrated that both strategies promoted regression in the 10-mm segments that were determined to harbor the greatest plaque burden at baseline. Overall, this result suggested that use of an intensive lipid lowering strategy has the potential to alter the natural history of progression of coronary atheroma. This corresponds with the recently reported Treating to New Targets (TNT) study which demonstrated that incremental LDL lowering with high dose compared with low dose atorvastatin was associated with a 22% reduction in the combination of clinical end points.
The beneficial effect of statin therapy on plaque burden was recently supported by the finding that in a small cohort of 40 males with hypercholesterolemia and ischemic heart disease that 3 months of a lipid-lowering diet followed by simvastatin 40 mg daily resulted in a 42.6% reduction in LDL and associated 6.3% reduction in plaque volume, consistent with plaque regression.55
While there appeared to be a continuous relationship between the change in LDL and change in plaque progression with both strategies in REVERSAL, at any level of LDL-C the regression line was lower for atorvastatin. It appeared that the lower progression rate with atorvastatin was equivalent to an additional 20% LDL-C lowering with pravastatin and suggested strongly that the beneficial properties of atorvastatin may extend beyond lipid lowering. Subsequent analysis demonstrated a correlation between the degree of CRP lowering and rate of progression of plaque, with subjects receiving the largest reduction in CRP demonstrating evidence of atheroma regression.56 The incremental benefit of intensive lipid lowering and its correlation with the degree of CRP reduction supported the recently reported benefit of high dose atorvastatin compared with pravastatin therapy on clinical event rates in patients with an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) study.57,58
LDL lowering was demonstrated to have a beneficial impact on plaque burden when commenced in the setting of a recent acute coronary syndrome. In the ESTABLISH study59 70 patients with ST elevation myocardial infarction, who proceeded to percutaneous intervention of the culprit lesion were randomized to receive atorvastatin 20 mg daily or conventional treatment with a lipid lowering diet for 6 months. A cholesterol absorption inhibitor could be initiated in the conventional arm if their LDL remained greater than 150 mg/dL. Forty eight of the subjects underwent serial IVUS examinations either proximal or distal to the intervention site at baseline and follow-up. Atorvastatin treatment was associated with a 41.7% reduction in LDL levels and promoted plaque regression, as evidenced by a 13.1% reduction in plaque volume. In contrast, LDL did not change in the conventional treatment group, which demonstrated an 8.7% increase in plaque volume. The percentage change in plaque volume correlated with both the LDL level at follow-up and the percent reduction in LDL. The result of this short term study is parallel to the finding in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) study60 which enrolled patients with acute coronary syndromes. In this study high dose atorvastatin was associated with a significant 16% reduction in clinical events within 16 weeks of an acute coronary syndrome.
IMPACT OF BLOOD PRESSURE LOWERING ON PLAQUE BURDEN
Clinical IVUS studies have also assessed the impact of targeting risk factors beyond plasma lipoproteins. There is currently no consensus view with regard to optimal blood pressure management in normotensive patients with established coronary artery disease. The recently reported Comparison of Amlodipine versus Enalapril to Limit Occurrences of Thrombosis (CAMELOT) study61 was designed to assess the influence that blood pressure reduction had on clinical events and plaque burden in normotensive subjects. A total of 1991 patients with coronary artery disease and a diastolic blood pressure less than 100 mm Hg, with or without treatment, were randomized to receive either amlodipine 10 mg daily, enalapril 20 mg daily, or placebo for 24 months. Treatment with amlodipine resulted in a 31% reduction in the composite incidence of clinical events compared with placebo. This benefit resulted largely from a 27% reduction in coronary revascularization and 42% reduction in hospitalization for angina. Two hundred and seventy four of these subjects participated in an IVUS substudy, which demonstrated plaque progression with placebo and no change in atherosclerotic burden with administration of amlodipine. When patients with blood pressure at baseline greater than the mean were considered (a prespecified analysis), the difference between the amlodipine and placebo groups became statistically significant. It was also noted that the effects of the treatment arms on clinical events corresponded with their effect on plaque burden.
USE OF SERIAL IVUS TO MONITOR PROGRESSION OF OTHER VASCULAR PATHOLOGIES
IVUS has provided a number of insights into the in vivo response of the arterial wall to angioplasty. In particular, it has established that restenosis following percutaneous non-stent interventions results from a combination of arterial recoil, remodeling and the development of neointimal hyperplasia.62 In addition to assessing the impact of bare metal and drug eluting stents,63,64 serial IVUS examinations have been employed to investigate the clinical utility of a number of experimental strategies that potentially inhibit neointima formation. In the multivitamins and probucol study group, 317 patients were randomised to receive the antioxidant probucol, multivitamins, the combination or placebo 30 days prior to coronary angioplasty and for a period of 6 months after. Serial IVUS performed in a subset of 94 patients confirmed that restenosis resulted primarily from inadequate remodeling in the setting of neointima formation, which was prevented by treatment with probucol.65 It was demonstrated that a cross-sectional narrowing less than 67% on IVUS performed immediately following angioplasty predicted a low rate of restenosis in patients treated with probucol at 6 months.66 Further, the experimental anti-inflammatory and antioxidant agent AGI-1067 was recently demonstrated to prevent restenosis following angioplasty, in the absence of QTc prolongation seen with probucol.67
As IVUS has emerged as the gold standard for the clinical surveillance of the development and progression of coronary artery vasculopathy in heart transplant recipients it provides a unique opportunity to assess the efficacy of strategies designed to inhibit this process. The onset of transplant vasculopathy portends a poor clinical outcome and is the leading indication for repeat cardiac transplantation.68 Recent reports have found that the progression of coronary vasculopathy predicts the long-term incidence of clinical events.69,70 Unfortunately there are few therapeutic options that have been demonstrated to have any impact on this process.71 It has become increasingly apparent that a cascade of immunological events promotes the formation of neointimal hyperplasia.72 As a result, immunological mediators have received increasing attention as potential targets.
Everolimus is a novel proliferation inhibitor and immunosuppressant.73 Serial IVUS was used to demonstrate the ability of everolimus to prevent the development of vasculopathy.74 six hundred and thrity four primary heart transplant recipients were randomised to receive 1.5 mg of everolimus, 3 mg of everolimus or 1-3 mg of azathioprine in combination with standard medical therapy. Coronary artery IVUS studies were performed within 6 weeks of transplantation and at 12 month follow-up. Compared with the azathioprine group, the incidence of vasculopathy was reduced by 33% and 42% with low and high dose everolimus respectively. Further, everolimus inhibited the increase in average maximal intimal thickness by 60%-70%. This benefit was associated with a 22%-42% reduction in the composite clinical endpoint of death, graft loss or retransplantation, loss to follow-up, acute rejection grade 3A or rejection with hemodynamic compromise. This result reflects an important ability of serial in vivo IVUS imaging to correlate the impact of therapeutic interventions on the incidence of both arterial vasculopathy and clinical events.
Although it is not a proof, all of the data from trials using IVUS and clinical information support the concept that IVUS can be used as a reliable representative of clinical outcome.
NEED TO DEVELOP STRATEGIES TO MONITOR PLAQUE ACTIVITY
It has become increasingly recognized that the composition of atherosclerotic plaque, in addition to its total burden, is an important determinant of its translation to clinical ischemia. Several groups have reported that myocardial infarction occurs predominantly in the vascular territory supplied by a culprit lesion, that is only mild to moderately stenotic on coronary angiography.10-12 Pathologic studies have demonstrated that macrophage rich plaques are more likely to undergo erosion or rupture of the fibrous cap, the typical precipitant of ischemic events.28,75 In support, it has been reported that systemic levels of CRP predict outcome in both healthy subjects76 and in the setting of acute coronary syndromes.77 Immense interest has therefore focused on the development of imaging modalities that monitor the activity of atherosclerotic plaque. A number of techniques have been utilized in the research setting to characterize plaque composition. While IVUS provides an excellent assessment of atherosclerotic burden, its ability to characterize plaque components is relatively limited to a broad distinction between echolucent (lipidic), echodense (fibrous), and calcific components. Although the relationship between a plaque's histological and ultrasonic characteristics are inconsistent.14 Recent developments of IVUS that have focussed on the analysis of radiofrequency backscatter allow for the characterization of plaque components, with good histopathological correlation.78 Spectral analysis of this backscattered data has been demonstrated ex vivo to accurately identify the size and composition of different components of atherosclerotic plaque in human coronary arteries. As a result, this modality has become available for use in the clinical setting to identify lipid rich, and therefore potentially vulnerable, lesions. Optical coherence tomography (OCT), which measures reflected light, rather than ultrasound, has been demonstrated to image atheroma macrophage activity with high resolution.79,80 In addition, the ability of catheters to quantify plaque temperature81,82 and compressibility81,83 has the potential to identify inflamed and lipidic regions. The major limitation of each of these strategies is their need for invasive cardiac catheterisation. The ideal approach would involve a non-invasive assessment of the coronary arteries. Magnetic resonance has been reported to characterize plaque components.84,85 However, an MRI technique for clinical use is not yet available. Computerized tomography (CT), in contrast, is limited at this point in time to the assessment of the extent of coronary calcification, detection of luminal stenoses and characterization of arterial remodelling in response to plaque.86-88 There are efforts to assess the arterial wall characteristics by using Hounsfeld units. The combination of CT with positron emission tomographic (PET) imaging, which has been demonstrated to accurately assess plaque macrophage activity,89 is promising. The further developments of these imaging techniques will provide an ideal opportunity to assess the serial response of plaque composition, and therefore vulnerability, in response to a number of therapeutic interventions in vivo.
CONCLUSION
The development of IVUS has enhanced our understanding of the factors that influence the progressive accumulation of atherosclerotic plaque. The ability to perform IVUS on a serial basis provides a unique opportunity to characterize changes in atheroma in response to various pharmacologic strategies. It has become apparent that aggressive modification of traditional cardiovascular risk factors can have a profound impact on the natural history of plaque progression. This benefit appears to complement the ability of these interventions to prevent clinical events. The ability to identify strategies that promote the regression of atheroma, rather than just halting progression or reducing plaque vulnerability, represents a major paradigm shift in cardiovascular protection. As a result, it will become increasingly important to incorporate serial assessment of plaque burden as a major endpoint in the design of future clinical studies that focus on the development of emerging strategies to further reduce cardiovascular risk.
Correspondence: Dr E. Murat Tuzcu.
The Cleveland Clinic Foundation. Department of Cardiovascular Medicine/F15
9500 Euclid Avenue, Cleveland OH 44195, USA.
E-mail: tuzcue@ccf.org