Several aortic valve sparing techniques have been described for the treatment of aortic root aneurysms. We report our experience using the reimplantation technique in 120 patients.
MethodsBetween March 2004 and October 2010, 120 patients with aortic root aneurysms underwent David operations. Of these, 51 were diagnosed with Marfan syndrome. Mean patient age was 31±12 years. The mean diameter of the sinuses of Valsalva was 51±5mm and moderate/severe aortic regurgitation was present in 16% of these patients. In the other 69 patients mean age was 56±14 years, the mean diameter of the sinuses of Valsalva was 53±7mm and moderate/severe aortic regurgitation was present in 66%. A bicuspid aortic valve was presented in 14 cases.
ResultsHospital mortality was 1.7%. Mean follow-up was 37±21 months; 94% of the patients survived and 96% had an aortic regurgitation below grade II during 5 years of follow-up. One patient required re-operation because of severe aortic regurgitation. No endocarditis or thromboembolic complications have been documented, and 96% of the patients did not receive any anticoagulation therapy.
ConclusionsShort- and mid-term results with the reimplantation technique for aortic root aneurysms are excellent. This technique prevents the need for chronic anticoagulation treatment as well as the complications arising from mechanical prostheses, and it should be the treatment of choice for young patients.
Keywords
.
INTRODUCTIONAortic root aneurysms frequently occur due to cystic medial degeneration, which is histologically presented as a loss of smooth muscle cells and degeneration of elastic fibers. These alterations are characteristic of the Marfan Syndrome (MS), although they can also be observed in patients with bicuspid aortic valves. Less frequently, aortic root aneurysms are found in elderly patients with degenerative diseases, especially atherosclerotic conditions.1
Annuloaortic ectasia and increased sinuses of Valsalva (SV) diameter both alter the coaptation surface of aortic leaflets and can cause aortic regurgitation (AoR) even if the leaflets have normal anatomy. These conditions can also cause aortic dissection and/or rupture. Surgical treatment is complex, and initial mortality from surgical procedures for this condition is high. In 1969, Bentall and De Bono2 described a surgical technique that, after the “button” modification described by Kouchoukos,3 has been the most widely used treatment for aortic root aneurysms, as it is reproducible, safe, and long lasting. This procedure requires aortic valve replacement, usually with a mechanical prosthesis, and as such is accompanied by lifelong anticoagulant treatment.
To avoid complications associated with a prosthesis and chronic anticoagulation treatment, especially in young patients, several techniques have been developed since the 1990s that allow for preservation of the patient's aortic valve. Two main types of treatment exist: the “remodeling” technique described by Yacoub,4 and the “reimplantation” technique described by David.5 David's technique yields greater stabilization of all components of the aortic root, and so is currently considered to be the method of choice in patients with annuloaortic ectasia.6, 7
This is a description of our experience with this technique in 120 patients, analyzing the surgical and follow-up results of our patients with regards to survival and the durability of the repair.
METHODSBetween March 2004 and October 2010, 120 patients with aortic root aneurysms underwent valve sparing surgery using the technique described by David.5 According to Gante's criteria,8 43% of our patients had MS. The clinical characteristics of the two groups (MS and non MS) are summarized in Table 1. The mean logistic EuroSCORE was 4.65±1.35 for the whole study, with no differences observed between groups.
Table 1. Clinical Characteristics of Both Groups.
| MS (n=51) | Non MS (n=69) | |
| Male | 35 (68.6) | 57 (82.6) |
| Age (years) | 31 ± 12 | 56 ± 14 |
| NYHA I-II | 50 (98) | 66 (95.6) |
| AHT | 2 (3.9) | 7 (10.1) |
| DM | 1 (1.9) | 5 (7.2) |
| DL | 2 (3.9) | 7 (10.1) |
| SV diameter (mm) | 51 ± 5 (42-70) | 53 ± 7 (42-75) |
| AoR grade III-IV | 8 (16) | 45 (66) |
| LVEF (%) | 60 (55-65) | 60 (53-64) |
| Aortic dissection | 0 | 0 |
| Bicuspid valve | 0 | 14 (20.2) |
AHT, arterial hypertension; AoR, aortic regurgitation; DL, dyslipidemia; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; MS, Marfan syndrome; NYHA, New York Heart Association; SV, sinuses of Valsalva.
Data are expressed as n (%), mean ± standard deviation (range), or mean (range).
In MS patients (n=51), surgery was indicated when the aortic diameter in the SV were >45mm (measured using echocardiogram as the distance between the internal margins of the aortic wall, or using computerized tomography) or when a progressive growth of the diameter was observed >2mm per year, and the anatomy of the aortic leaflets and the rest of the aortic root was favorable (normal leaflets, with no calcification or multiple fenestrations). In the other 69 patients, the procedure was indicated when the diameter of the SV was ≥55mm (50mm in patients with bicuspid valve), and 25 patients who underwent surgery had severe AoR along with diameters <50mm. We performed an intraoperative transoesophageal echocardiogram on all patients in order to evaluate the aortic valve.
Since 2004, we processed the following number of cases per year: 11, 14, 16, 20, 20, 24, and 15; therefore, 20% of the total study sample has complied with a 5-year follow-up program.
This treatment was elective in all patients. The Hospital 12 de Octubre's ethics committee approved our study, and all patients gave informed consent to use their clinical information.
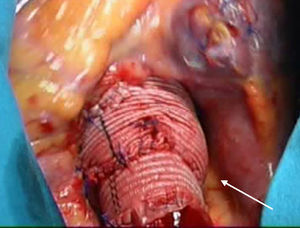
Surgical TechniqueWe used a median sternotomy as the surgical access point. In all cases, we established extracorporeal circulation (ECC) and induced moderate hypothermia (32°C), protecting the myocardium by intermittent antegrade and retrograde blood cardioplegia infusion every 20min. After clamping the aorta, the ascending aorta was transected and the segment with the aneurysm was resected all the way to the valve, conserving a 2mm-3mm segment of the aortic wall for later reimplantation of the aortic valve into the tubular prosthesis. We dissected the coronary buttons using the technique described by Kouchoukos et al.3 We used the David type I technique5 in 29 patients (using a Gelweave Valsalva graft in 28 cases [Sulzer Vascutek; Renfrewshire, United Kingdom], and a straight tubular Dacron graft [Hemashield] in one case). In the other 91 patients, we used the modification described by Miller.9 This technique consists of interpositioning two tubular Dacron grafts (Hemashield) of different sizes in order to shape them to the aortic root (Figure 1). The proximal graft was 32mm to 34mm, and was reduced using single sutures at the level of the aortic ring in order to achieve the correct size. To achieve a proper sinotubular junction, we created an anastomosis between this graft and a 26mm to 28mm smaller implant placed distally (Figure 1). Diameter was estimated in the first few cases using the formula proposed by David5: D=[(h×2)×0.67]+2×Ao; where D is the diameter of the graft, h is the mean height of the leaflets, and Ao is the thickness of the aortic wall (all measurements made in millimeters). We have determined that this diameter is equivalent to that obtained by applying upwards traction to the commissures, elevating them to the point in which the leaflets coapt sufficiently, which corresponds to the diameter of the aortic ring and the sinotubular junction. This is the method that we currently use, and which we have published previously.10
Figure 1. Surgical area. Image of the aortic root following reconstruction with a double velour Dacron graft. Morphology of the new sinuses of Valsalva and the sinotubular junction.
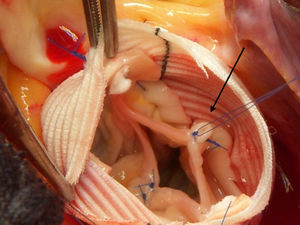
Prolapsed aortic leaflets were corrected after performing the valve reimplantation in 29 patients, which involved the following techniques: plication of the free edges at the Arantius’ nodules in 22 patients (Figure 2), subcommissural plasty in 15 patients, free edge reinforcement using GoreTex 7/0 sutures (W.L. Gore and Associates; Flagstaff, Arizona, USA) in 3 patients, and detachment of the raphe from bicuspid valves in 2 patients. Other procedures associated with aortic root surgery were mitral valve repair in 9 patients, tricuspid valvuloplasty in 4, myocardial revascularization in 6, and closure of the atrial septal defect or foramen ovale in 14 patients.
Figure 2. Surgical area. Image of the aortic leaflets after correction of the prolapse using free edge plication with prolene 6/0 sutures.
Follow-upClinical follow-up started 2 months after surgery, and continued on a yearly basis. We performed a transthoracic echocardiogram before discharging the patient from the hospital, at 2 months, and then annually. Aortic regurgitation was semi-quantitatively rated as slight, moderate, or severe. Follow-up sessions were made over the telephone with patients from outside of Madrid, and they sent their corresponding echocardiogram reports for analysis. No patients dropped out during the follow-up period, which lasted a mean of 37±21 months (range: 1-80).
RESULTSMean duration on ECC and aortic clamps was 163±46min (range: 96-355) and 137±33min (range: 90-275), respectively. Four patients required conversion to mechanical aortic prosthesis implantation due to significant residual AoR observed in the intraoperative transesophageal echocardiogram.
Overall hospital mortality was 1.7% (2 patients). The first, a 7-year-old male with Beals syndrome, died from refractory low cardiac output following the operation. In the postoperative coronary angiography, we observed irreversible spasms of the descending anterior and right coronary arteries. The second case was a 60-year old male with no initial postoperative complications, and who was discharged from the hospital 9 days after the operation, but was readmitted 5 days later with fever. The transesophageal echocardiogram indicated a normally functioning aortic valve, with no evidence of infectious endocarditis or pericardial effusion. Six days later, the patient suffered cardiac arrest of an unknown cause. The family did not give authorization for a necropsy to determine cause of death.
Three patients required post-operative surgical exploration due to bleeding in the first hours following the procedure. Three patients suffered perioperative acute myocardial infarction with no hemodynamic repercussions, associated with myocardial revascularization in 2 patients with coronary lesions. Two patients were treated for transitory neurological dysfunction, and 1 patient required a definitive pacemaker implant due to an atrioventricular block. Table 2 shows the rates of postoperative complications for both groups.
Table 2. Postoperative Complications.
| Complication | MS (n=51) | Non MS (n=69) |
| Conversion | 2 (3.9) | 2 (2.8) |
| AMI | 0 | 3 (4.3) |
| Definitive pacemaker | 0 | 1 (1.4) |
| Re-operation for bleeding | 3 (5.8) | 0 |
| TND | 0 | 2 (2.8) |
| CVA | 0 | 0 |
| Hospital mortality | 0 | 2 (2.8) |
AMI, acute myocardial infarction; CVA, cerebrovascular accident; MS, Marfan syndrome; TND, temporary neurological dysfunction.
Data are expressed as n (%).
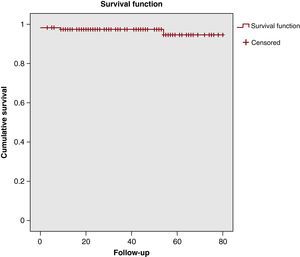
Two patients died during the first year following surgery: 1 due to a ruptured aneurysm of the abdominal aorta, and 1 due to pulmonary thromboembolism. Only 1 patient was re-operated on for severe AoR during follow-up, 5 years after the surgery. Another patient was operated on a second time due to severe mitral regurgitation. No other subsequent operations were required for other causes, and no cerebrovascular events were recorded. In the last follow-up visit, 85% of patients were off the anticoagulation medication, and 96% had an AoR grade ≤II (87% of patients had no AoR or grade I). Survival was 97±1.5%, 97%±1.5%, and 94±3.1% at 1, 3, and 5 years follow-up (Figure 3).
Figure 3. Patient survival curve. Survival at 1, 3, and 5 years: 97±1.5%, 97%±1.5%, and 94±3.1%.
DISCUSSIONThe use of valved conduits for the surgical treatment of aortic root aneurysms has demonstrated very positive mid- and long-term results,11, 12 and continues to be a safe and reproducible technique. However, inherent complications do arise when using mechanical aortic prostheses. The annual incidence of thrombotic and/or hemorrhagic complications due to chronic anticoagulation treatment is estimated at 2% to 4%.13, 14 Furthermore, the need for chronic medication implies severe limitations to the quality of life in young patients, and this treatment creates difficulties for managing pregnancies in women.
As such, new techniques were developed in the 1990s that attempt to preserve the aortic valve in order to avoid the inconveniences associated with mechanical prostheses. The aortic valve reimplantation technique described by David5 has achieved the best results in durability, due mainly to the fact that all components of the aortic root (ring, SV, and sinotubular junction) are stabilized with this technique. David et al.15 produced excellent results: freedom from severe and moderate AoR was 94%, 95% of patients did not require a second operation, and survival at 10 years was 92%. Kallenbach et al.16 published a study with 284 patients and a mean follow-up period of 41 months. At the final follow-up session, 96% of patients had good functional class and the rate of re-operations was 5%. Svensson et al.,17 with 129 patients, recorded a survival rate of 99% at 5 years.
The aortic root remodeling technique described by Yacoub4 is also used to treat aortic root aneurysms. Aicher et al.18 published their experience using this method with 274 patients, all of which had nondilated aortic rings: 96% of patients did not require another operation in 10 years and 98% did not require aortic valve replacement. In spite of the good results published by various authors with this technique, it does not stabilize the aortic ring, which predisposes patients with aortic annuloaortic ectasia to a greater probability of recurrent AoR. In the results published by David et al.,6 94% of patients undergoing reimplantation did not have severe or moderate AoR after 10 years of follow-up, whereas the rate was 75% for the remodeling technique. Cameron et al.19 followed 65 patients for 5 years that had undergone either remodeling surgery or aortic valve reimplantation, and observed that 9 of the 10 patients that suffered severe late AoR had undergone remodeling, and 8 of these cases were due to dilation of the valve ring.
Our study with 120 patients undergoing aortic valve reimplantation is one of the largest published, and the results obtained are comparable to those from other institutions with experience using this method.15, 16, 17 In 2007, we presented our initial experience preserving the aortic valve,20 which primarily focused on the early results from a very specific sample population (18 patients with MS). Although initial morbidity and mortality rates are important, the fundamental aspect of these methods that must be analyzed is the functioning of the aortic valve and the mid- and long-term absence of the need for re-operations. The early results, durability of the repair made, and the absence of significant AoR after a follow-up period of 37±21 months have been very satisfactory. Moreover, we published the entire study, including 14 patients with bicuspid aortic valves, and 29 cases in which the leaflets required some kind of intervention.
In all cases, except for the first patient, we attempted to re-create the SV. We believe that reconstructing the SV improves the functioning of aortic leaflets and allows for more physiological closing and opening, which theoretically would increase the durability of the repair made. To this end, we used the modification described by Miller.9 With this method, it is far more simple to implant the aortic valve, given the larger diameter of the graft used. In addition, this procedure allows the surgeon to modify the different parts of the aortic root, adapting the size of the ring, the height of the commissures, and the diameter of the sinotubular junction in each case and with no additional limitations. This would not be possible when using a preformed prosthesis, because the appropriate height of the commissures does not always coincide with that provided for by the graft. This method also allows for size matching between the tubular graft and the native aorta at the distal anastomosis,21 which is a very important concept, especially in MS patients with a significantly reduced diameter of the distal ascending aorta.
Surgery must be performed on prolapsed or defective aortic leaflets when required. Recent studies have shown that aortic valve repair is a very efficient and durable technique. De Kerchove et al.22 published their results after correcting prolapsed aortic leaflets in 88 patients. They compared their results from repairing leaflets with plication of the free edges using polypropylene sutures or reinforcing the free edges using GoreTex sutures. After 5 years of follow-up, re-operations were not required in 100% of patients with free edge plication, 96% of those with reinforcement, and 93% of those undergoing both techniques. The absence of severe or moderate AoR at 3 years was 100%, 92%, and 89%, respectively. In a study with 427 patients who underwent surgery to correct prolapsed leaflets, Aicher et al.23 observed an absence of AoR ≥ grade II after 5 years of follow-up in 92% of patients with free edge plication, 90% of those with triangular resections, and 90% of those with pericardial patch.
In our study, prolapsed aortic leaflets were corrected after valve reimplantation in 29 patients using some of the techniques described in Methods. Subcommissural plasty was also used in 13 of these patients to increase the coaptation surface area of the leaflets. In all cases, we achieved a leaflet coaptation surface height (as measured using intraoperative transesophageal echocardiogram) >8mm, as recommended by other authors.18 No patient undergoing surgery for aortic leaflets suffered from regurgitation during the follow-up period. We recommend operating on the leaflets after completing the valve reimplantation, as the altered geometry of the aortic root could cause prolapse of previously normal leaflets. The simplest technique with the best results is plication of free edges, which entails dissecting and elevating the leaflets, thus preventing prolapse.
Aortic valve sparing appears to be especially important in MS patients. Volguina et al.24 conducted a multicenter prospective study of 151 patients undergoing surgery at 18 European and United States hospitals with experience in the treatment of MS. They compared preservation methods and aortic root replacement. This study produced two interesting conclusions: first, that aortic valve reimplantation using the David type V method is the most commonly used procedure, and second, the early results from the study were similar to those obtained using the Bentall method, in spite of it being a more complex procedure. Cameron et al.7 described their long-term results from a study with 372 MS patients undergoing surgery over a 30-year period (1976-2006). The Bentall procedure was used in 269 patients, and valve sparing techniques in 85 patients (1998-2006). Not a singe case of hospital mortality was recorded for the 327 patients who underwent elective surgery. Late death occurred in 74 patients, and the most frequent cause of death was dissection or rupture of the residual aorta (70 in the Bentall group, and 2 in the valve sparing group). The thromboembolic complications that developed during the follow-up period occurred in patients with valved conduits (4 patients developed graft thrombosis, 15 suffered embolic events, and 14 suffered cerebral events). The absence of thromboembolism in study patients was 96.3%, 93.3%, 91%, and 89.8% at 5, 10, 15, and 20 years, respectively. Forty patients underwent valve sparing using the technique developed by Yacoub4 (7 patients developed grade 3-4 AoR late in the follow-up period), and in 44 patients we used the David I technique5 with preformed sinuses (no patients from this group developed AoR during the follow-up period). Currently, valve sparing techniques are the most commonly used by this group. In MS patients, surgery is suggested at an early age, and many of these patients will probably require re-operation due to altered collagen fibers. In these patients, the lack of continued chronic anticoagulation treatment would avoid a severe risk in the long term.
According to the most recent clinical guidelines published by the American Heart Association25 for the diagnosis and treatment of patients with thoracic aortic disease, class I indications for aortic root aneurysm surgery in asymptomatic patients has been established at diameters ≥55mm, although this could be as low as 40mm to 50mm in patients with MS or a bicuspid valve, depending on other factors. Given the excellent early results and the durability of the repair made, any patient with MS and an SV diameter >45mm is a candidate for this surgery at our hospital. Early surgery for these patients is the best way to avoid deterioration of the aortic valve, since SV diameters >5.5cm imply very thin and elongated leaflets, and at this point the results of a repair are questionable.
CONCLUSIONSAortic valve reimplantation offers excellent short- and mid-term results in terms of the need for a re-operation and AoR recurrence. It also eliminates the complications associated with valve prostheses and anticoagulant treatments, and so should be considered as the treatment of choice for aortic root aneurysms in young patients.
CONFLICTS OF INTERESTNone declared.
Received 3 December 2010
Accepted 12 February 2011
Corresponding author: Servicio de Cirugía Cardiaca, Hospital Universitario 12 de Octubre, Avda. de Andalucía s/n, 28027 Madrid, Spain. aforteza.hdoc@salud.madrid.org