Aortic stenosis is a common disease that is more prevalent in individuals older than 75 years.1 Surgical valve replacement is the standard approach for symptomatic patients with severe aortic stenosis.2 Until a few years ago, the imaging techniques to be employed prior to surgical valve replacement were well established: transthoracic or transesophageal echocardiography to confirm the diagnosis and define the severity of the stenosis (based on pressure gradients, aortic valve area determined by planimetry, etc.) and the details of the valve anatomy (including valve calcification, etc.). The diameters of the aortic root and ascending aorta were evaluated, and the left ventricular ejection fraction was defined.3 Computed tomography and magnetic resonance imaging allow data to be gathered on additional aspects of the aortic anatomy when necessary. Preoperative imaging is used to establish the indication for surgery and to evaluate details of the anatomy. Visualization of valve involvement at surgery confirms noninvasive findings. Specifically, the aortic annulus is evaluated using dilators to accurately determine the correct valve size.
A significant percentage of patients with severe aortic stenosis and comorbidities are not candidates for surgery because of the high risk of surgical mortality; thus, a less invasive technique was developed for their treatment: transcatheter aortic valve replacement (TAVR) or transcatheter aortic valve implantation (TAVI). This novel therapeutic modality has been employed in more than 50 000 patients around the world, and its efficacy has been verified in follow-up studies of up to 5 years’ duration.4 One characteristic of this procedure is that the operator has no direct view of the aortic root or valve during the intervention; therefore, the greatest possible amount of information must be obtained before the performance of this technique. Multidetector computed tomography (MDCT) provides relevant information for: a) proper candidate selection; b) defining the most appropriate valve size for the patient; and c) identifying the anatomic factors associated with complications.5
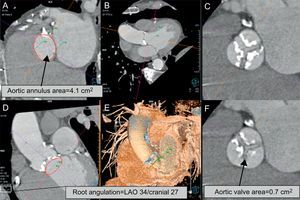
Undoubtedly, one of the most important tasks to be carried out before placement of a percutaneous aortic valve is accurate determination of the diameter of the aortic annulus. The aortic annulus is the base of the aortic root; it represents a transitional area between the left ventricular outflow tract and the aorta and, moreover, is the structure in which the valve is secured. In most patients, it has an elliptical shape, and 2-dimensional imaging techniques such as echocardiography or angiography usually underestimate its true dimensions as they measure the diameter in a single plane. In the measurement of the aortic annulus, MDCT and magnetic resonance imaging offer greater accuracy as they enable 3-dimensional evaluation of it size, allowing measurement of the largest and smallest diameters, the true area of the annulus, and the diameter derived from the measurement of the area. The sizing of the aortic annulus with these 2 methods exhibits low intraobserver and interobserver variability, which is important since the accuracy of these measurements improves selection of the proper diameter of the prosthesis to be implanted (Fig. 1).6,7
Most important measurements made in the aortic valve and annulus. A: Image reconstructed at the level of the aortic annulus; the diameter and area of the annulus are measured. The angiographic image, when reconstructed in a modern workstation, shows the angulation corresponding to the preceding image (B, D, and E). This information is used as a guide during transcatheter aortic valve implantation. C and F: Severity and extent of valve calcification and a markedly reduced aortic area. LAO, left anterior oblique.
One of the additional advantages of performing MDCT prior to TAVR/TAVI is that this technique it allows the characteristics of the aortic root to be evaluated in detail, since it provides information on important aspects such as the distance between the valve plane and the origin of the coronary arteries, the dimensions of the aortic root, and the presence, severity and extent of valve calcification.8 These data define the inclusion and exclusion criteria for each of the different types and sizes of valves, and identify patients at higher risk for complications (annulus rupture or the need for pacemaker placement in patients with severe calcification or acute myocardial infarction due to embolization of calcified plaques near the origin of the coronary arteries).9 Moreover, 3-dimensional reconstruction of tomographic images allow determination of the angulation of the valve plane that correlates with the angiographic projections employed during valve implantation, a factor that can reduce the time and radiation required during the procedure.10
ACCESS SITEVascular lesions at the access site were one of the most common complications in the initial experience with TAVR/TAVI.11 Multidetector computed tomography provides adequate visualization of the vascular and cardiac structures to aid selection of the best access site for valve implantation. This technique allows the diameter and degree of calcification of the iliac and femoral arteries to be determined, as well as their course and tortuosity. Small, calcified, and tortuous arteries increase the risk of vessel dissection and perforation, and are frequent contraindications for peripheral access. The diameter and calcification of the ascending aorta are evaluated, as are those of the axillary and subclavian arteries. Moreover, the characteristics of the left ventricular apex, including the presence of apical thrombus, and its distance from the chest wall are analyzed to allow planning of a transapical approach.12
DISADVANTAGES AND LIMITATIONSOne of the disadvantages of using MDCT is that patients are exposed to radiation; however, this is less important in the population of advanced age that is currently evaluated for TAVR/TAVI. A limitation that has greater clinical relevance is the need to use a contrast medium, which is associated with a risk of deterioration of the glomerular filtration rate. Potential alternatives are protocols involving a reduction in the amount of contrast medium (for example, intra-arterial injection for the pelvic vessels) and studies that do not require contrast media.
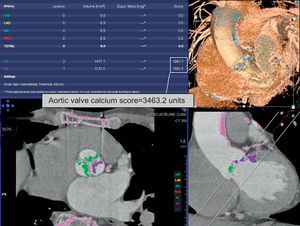
FUTURE PROSPECTSA subject of current interest is evaluation of patients with severe aortic stenosis who have low-pressure gradients and preserved left ventricular function; in these patients, MDCT can provide an accurate determination of the valve area. This technique can also provide a correlation of the valve calcification and the severity of stenosis when there are doubts about the latter (Fig. 2).13,14 Consequently, there is now one more tool to add to recent proposals for the evaluation of these patients.15,16
CONCLUSIONSThe development of percutaneous valve replacement has had a profound impact on cardiovascular imaging. Preoperative MDCT provides relevant information for decision making prior to, during, and probably after the procedure. This diagnostic modality has become established as a standard tool in world-renowned TAVR/TAVI centers. As this therapeutic modality evolves, important developments in the imaging techniques used in its evaluation are expected as well.
CONFLICTS OF INTERESTNone declared.