The subcutaneous implantable cardioverter-defibrillator (S-ICD) has emerged as an alternative to the transvenous defibrillator. The incidence of complications is similar, with inappropriate shocks (IS) being more frequent than those occurring with contemporary programming of transvenous defibrillators. Several improvements have been implemented after the S-ICD was approved for use in Europe in 2009. This study reports the results of S-ICD use in a single center, whose experience began late, at the end of 2013.
MethodsProspective observational study including consecutive patients with defibrillator indication and no indication for either permanent pacing or cardiac resynchronization who underwent S-ICD implantation. Implant data and long-term follow-up were analyzed.
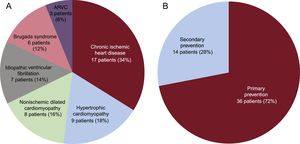
ResultsAn S-ICD was implanted in 50 patients who were deemed suitable after electrocardiographic screening. The mean age was 46.9±15 (range, 15-78) years and 72% were male. Thirty eight percent had left ventricular ejection fraction ≤ 35%. The most frequent heart disease was ischemic heart disease (34%), followed by hypertrophic cardiomyopathy (18%). The intermuscular technique was used, with 3 incisions in 10% and 2 incisions in the remaining 90%. Ventricular fibrillation was induced in 49 patients, with 100% effectiveness in their conversion. After a mean follow-up of 18.1 (range, 2.3-44.8) months, there were no late complications requiring surgical revision, the rate of IS was 0%, and 1 patient (2%) experienced appropriate shocks.
ConclusionsImprovements in technology, implant technique and device programming, along with appropriate patient selection, have led to outstanding acute and long-term results, especially regarding the absence of both IS and complications requiring surgical revision.
Keywords
To minimize patient risk from the endovascular leads used with conventional implantable cardioverter-defibrillators (ICDs) without impacting their effectiveness, an entirely subcutaneous ICD (S-ICD; Boston Scientific) system was developed. Although its initial results are promising, this device is not free of complications and its rates of inappropriate shocks (ISs) of between 5% and 25% in the first reported experiences1 are slightly higher than those seen with contemporary transvenous ICD programming, which can be less than 5%.2 The Spanish experience with the S-ICD is still scarce because the first such implantations were not performed until 2013, much later than in other European countries.3,4 Since the device became commercially available, and largely due to the accumulated experience, various improvements have been made to the implantation technique,5–8 programming,9 and even the device itself regarding its size and the incorporation of new software,10,11 which have all helped to reduce the incidence of problems related to this new device.
The present work describes the experience of a single center with the contemporary use of the S-ICD, which, due to its later introduction, had incorporated most of the above improvements from the beginning, permitting analysis of their role in reducing the incidence of complications.
METHODSThis prospective observational single-center study details our experience with the S-ICD in consecutive patients and reports data on the selected patients, preimplantation electrocardiographic screening, implantation technique, ventricular arrhythmia induction testing, follow-up, and complications occurring until the final revision in each patient. Two types of complications were distinguished: periprocedural, comprising those occurring during the implantation procedure or in the next 24hours; and delayed, comprising those occurring more than 24hours after the implantation.
PatientsFrom October 2013 to April 2017, patients were selected for S-ICD implantation if they had a standard indication for ICD therapy, namely, no indication for cardiac resynchronization therapy or permanent cardiac pacing. The reason why an S-ICD was chosen instead of a transvenous ICD varied during the inclusion period according to the latest evidence available on this new therapy in the literature.
The novel nature of this therapy and the lower levels of long-term evidence vs transvenous ICDs were explained to all selected patients. All patients signed an informed consent form before the implantation procedure.
DevicesThe S-ICD and its functioning have been detailed previously.12,13 Three S-ICD models were implanted: first, the Cameron S-ICD SQ-RX 1010 device (with a volume of 69.9mL and estimated longevity of 5.1 years), then the Emblem S-ICD A209, and then the Emblem MRI S-ICD A219 (these latter 2 have a volume of 59.5mL and estimated longevity of 7.3 years; the Emblem MRI S-ICD A219 has a new algorithm, SMART Pass, which activates a 9-Hz filter designed to reduce the amplitude of low frequency signals while maintaining an appropriate sensing margin, which improves detection in the case of high-amplitude T or P waves10; this algorithm was also available from the beginning for the Emblem MRI S-ICD A219 and was introduced to all Emblem S-ICD A209 devices via a software update in April 2016). Previously, in September 2014, another software update applied to implanted devices and to all subsequent implantations added an additional algorithm in the device certification phase (ACWADD algorithm) to reduce the possibility of T wave oversensing.11
Preimplantation Electrocardiographic AssessmentElectrocardiographic screening was performed to determine the ability of S-ICD implantation to reduce the risk of ISs due to inadequate signal detection. The S-ICD programming was only considered appropriate when the analysis was satisfactory in at least 1 lead or vector in both the dorsal decubitus and standing positions. The theoretical left and right parasternal lead location was assessed in all patients.
ImplantationImplantations were performed with local anesthesia and conscious sedation and analgesia or with local and general anesthesia and mechanical ventilation according to anesthesiologist availability. Prophylactic antibiotics were not used. Fluoroscopy was not used during the implantation procedures. The 3-incision technique was used initially but the 2-incision technique, which avoids the more cranial parasternal incision, was later adopted after demonstration of its simplicity and safety.7 Ventricular fibrillation induction testing was performed with the same device, with a programmed shock-only zone at 170 bpm and an initial 65-J shock with the S-ICD; if the first shock was ineffective, an additional 80-J shock with reversed polarity was attempted, followed by 300-J shocks with an external defibrillator. The device was programmed in 2 zones, a conditional discrimination zone for nonventricular arrhythmias and a shock-only zone.
Follow-upFace-to-face follow-ups were performed 3 months after the implantation and then every 6 to 12 months. In addition, using the Latitude system from Boston Scientific, remote follow-up was performed in patients who were implanted with second- and third-generation devices.
Statistical AnalysisData were analyzed using SPSS software (version 20.0; IBM Corp.; Armonk, New York, United States). Continuous variables are expressed as mean ± standard deviation and categorical variables as absolute values and percentages.
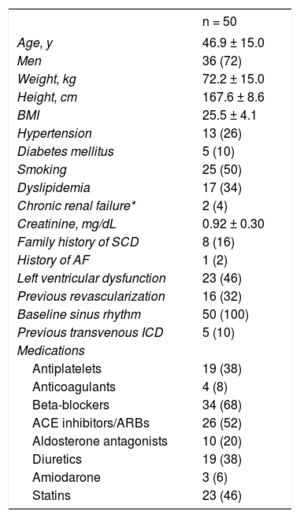
RESULTSPatient CharacteristicsIn total, 56 patients were selected for S-ICD implantation; 6 of these did not ultimately receive an S-ICD for the following reasons: 3 because the preimplantation electrocardiographic screening was unsuccessful, 1 because the patient opted not to undergo device implantation, 1 because the patient preferred a transvenous ICD, and 1 because the patient required permanent pacemaker implantation. The general characteristics of the remaining 50 patients are detailed in Table 1 and the type of heart disease and type of indication are shown in Figure 1. Initial data on the first 8 patients to receive an S-ICD in our center have already been published.13 The mean age was 46.9 ± 15 (15-78) years and the mean body mass index was 25.5 ± 4.1 (16.5-35.0). Slightly more than a third of patients (38%, 19 patients) had a left ventricular ejection fraction ≤ 35%. All patients were in New York Heart Association function class I or II. Five patients (10%) already had a transvenous ICD; the reasons for S-ICD implantation were defibrillation lead dysfunction in 2 patients and infection of the previous system in 3.
General Patient Characteristics
| n = 50 | |
|---|---|
| Age, y | 46.9 ± 15.0 |
| Men | 36 (72) |
| Weight, kg | 72.2 ± 15.0 |
| Height, cm | 167.6 ± 8.6 |
| BMI | 25.5 ± 4.1 |
| Hypertension | 13 (26) |
| Diabetes mellitus | 5 (10) |
| Smoking | 25 (50) |
| Dyslipidemia | 17 (34) |
| Chronic renal failure* | 2 (4) |
| Creatinine, mg/dL | 0.92 ± 0.30 |
| Family history of SCD | 8 (16) |
| History of AF | 1 (2) |
| Left ventricular dysfunction | 23 (46) |
| Previous revascularization | 16 (32) |
| Baseline sinus rhythm | 50 (100) |
| Previous transvenous ICD | 5 (10) |
| Medications | |
| Antiplatelets | 19 (38) |
| Anticoagulants | 4 (8) |
| Beta-blockers | 34 (68) |
| ACE inhibitors/ARBs | 26 (52) |
| Aldosterone antagonists | 10 (20) |
| Diuretics | 19 (38) |
| Amiodarone | 3 (6) |
| Statins | 23 (46) |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARBs, angiotensin II receptor blockers; BMI, body mass index; ICD, implantable cardioverter defibrillator; SCD, sudden cardiac death.
Values represent No. (%) or mean ± standard deviation.
Resting electrocardiographic screening was performed in all patients. The screening results are shown in Figure 2.
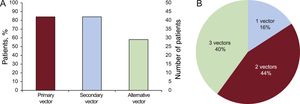
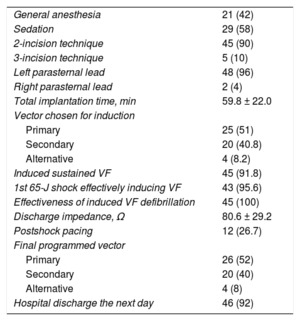
Implantation, Induction Testing, and Final Programming DataAll implantations were performed in the electrophysiology laboratory by 4 cardiologists with extensive implantation experience with all types of electronic cardiac devices. Only 1 of the patients was anticoagulated at the time of implantation, because this patient already had a mechanical tricuspid valve.14 The S-ICD was implanted satisfactorily in all 50 patients (100%), 2 with the lead in the right parasternal position (4%) and the remainder with the lead in the left (96%) (Figure 3). The first 10 patients (20% of the total) received a Cameron S-ICD SQ-RX 1010 device, the next 18 (36%) an Emblem S-ICD A209, and the final 22 (44%) an Emblem MRI S-ICD A219. The basic implantation characteristics are described in Table 2. Induction testing and ventricular fibrillation detection (Figure 4A) was performed in all patients except 1 (2%; due to left ventricular apical thrombus), with 100% effectiveness for the S-ICD; only 2 patients required a second maximum-energy shock (80J) with reversed polarity after an ineffective 65-J shock with standard polarity. The mean duration of ventricular fibrillation therapy was 16.7 ± 2.8 (13.4-26) seconds. A sustained ventricular arrhythmia was not achieved in 4 of the 45 patients (8.8%) who underwent this procedure, even after up to 3 attempts with up to 5seconds of continuous current with the device. Two zones were programmed with the following heart rate cutoffs: the conditional zone with thresholds of 220 bpm in 4 patients, 200 bpm in 37, 180 bpm in 8, and 170 in 1; and the shock zone with thresholds of 250 bpm in 45 patients and 240 bpm in 5. The most common programming was that of a conditional zone from 200 bpm and a shock zone from 250 bpm (36 patients, 72% of the total).
Implantation and Induction Testing Data
| General anesthesia | 21 (42) |
| Sedation | 29 (58) |
| 2-incision technique | 45 (90) |
| 3-incision technique | 5 (10) |
| Left parasternal lead | 48 (96) |
| Right parasternal lead | 2 (4) |
| Total implantation time, min | 59.8 ± 22.0 |
| Vector chosen for induction | |
| Primary | 25 (51) |
| Secondary | 20 (40.8) |
| Alternative | 4 (8.2) |
| Induced sustained VF | 45 (91.8) |
| 1st 65-J shock effectively inducing VF | 43 (95.6) |
| Effectiveness of induced VF defibrillation | 45 (100) |
| Discharge impedance, Ω | 80.6 ± 29.2 |
| Postshock pacing | 12 (26.7) |
| Final programmed vector | |
| Primary | 26 (52) |
| Secondary | 20 (40) |
| Alternative | 4 (8) |
| Hospital discharge the next day | 46 (92) |
VF, ventricular fibrillation.
Values represent No. (%) or mean ± standard deviation.
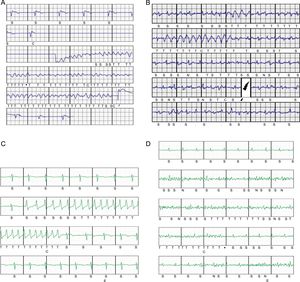
A: Postimplantation ventricular fibrillation induction with effective conversion to sinus rhythm with a 65-J subcutaneous defibrillator shock. B: Periprocedural inappropriate shocks due to rapid atrial fibrillation with intermittent wide complexes; dual-zone programming, conditional at 180 bpm and shock at 250 bpm. C: Nonsustained monomorphic ventricular tachycardia that triggered device therapy; dual-zone programming, conditional at 200 bpm and shock at 250 bpm. D: Episode classified as nonsustained ventricular tachycardia due to oversensing of nonphysiological signals generated by movement of the upper left extremity in a patient; dual-zone programming, conditional at 200 bpm and shock at 250 bpm.
In total, 96% of patients were free of periprocedural complications. Periprocedural complications occurred in 2 patients (4%). One patient, after induction of ventricular fibrillation that was correctly detected and effectively treated with the first shock and after wakening from the anesthesia and with dual-zone device programming (180 and 250 bpm), developed a state of intense agitation leading to rapid atrial fibrillation, with intermittent wide complex rhythms (aberrant or ventricular complexes); in addition, signal oversensing occurred and 2 ISs were delivered (Figure 4B), with sinus rhythm established once the agitation resolved. In another patient, minutes after pocket closure, a hematoma was detected in the generator pouch requiring immediate surgical wound revision; an internal active bleeding site was detected, which was cauterized without incident.
In all patients, chest radiography performed the next day confirmed the correct position of the system and no complications were observed. Most patients were discharged the following day; when not, it was for reasons unrelated to the implantation and none of these patients had been admitted originally for the procedure. There was no incidence of premature battery depletion. All patients who received a second- or third-generation device (40 patients, 80% of the total) were supplied with a Latitude monitor for remote in-home monitoring.
In total, 33 patients (66%) were followed up for more than 1 year after implantation. After a mean follow-up of 18.1 (2.3-44.8) months, only 1 patient (2%) showed ventricular arrhythmia episodes requiring appropriate device shocks and 3 (6%) were recorded to have had asymptomatic self-limiting ventricular tachycardia after receiving a shock (Figure 4C), detected prematurely upon activation of a Latitude system alert. Notably, there were no ISs during follow-up of the 50 patients (0%).
The initially programmed sensing vector was changed in only 1 patients, 18 months after implantation, due to noise that activated a shockless alert, which was reproduced upon movement of the left upper extremity (Figure 4D). One patient died (2% mortality rate) 10 months after the implantation due to noncardiac causes, a vesical neoplasm; the rate of late complication-free survival was 98% and no patient required surgical revision of the system. Three months after implantation, 1 patient (2%) developed an infection of the parasternal wound after the 2-incision technique, with positive cultures for Staphylococcus aureus; the infection resolved after intravenous antibiotic therapy.
DISCUSSIONMain FindingsThis single-center experience with the delayed introduction of S-ICD implantation shows highly favorable results, with an acceptable periprocedural complication rate and no late complications requiring surgical revision of the implanted system. Even more notable is the lack of ISs during follow-up, which exceeded 1 year in 60% of patients. In line with previous data, the therapy was 100% effective for induced ventricular arrhythmias.
Patient CharacteristicsSimilar to what was seen in the main series published on patients with S-ICDs,15–17 the mean patient age (46.9 years) was less than that typically seen in registries of patients who received an ICD, including a Spanish registry.18 To a large extent, the lower age was due to a higher percentage of patients with arrhythmogenic right ventricular cardiomyopathy, Brugada syndrome, or hypertrophic cardiomyopathy (38% of the total). These diseases are nonetheless associated with a higher risk of inappropriate therapies in patients with an ICD and, in relation to S-ICD specifically, are also associated with a higher frequency of worse detection of cardiac signal and potential dynamic changes in the QRS-T and, thus, an increased likelihood of ISs.19–22
Chronic ischemic heart disease was the most common underlying disease in this cohort (34%); 46% of patients had left ventricular systolic dysfunction and the rate of implantations in primary prevention was higher than that generally seen in Spain for ICDs,18 but was similar to that observed in the main S-ICD series.15–17 In a cohort of 856 patients with an S-ICD and after a mean follow-up of more than 600 days, Boersma et al.23 reported no differences in S-ICD performance according to whether the indication was primary or secondary prevention and, in primary prevention, it was independent of whether the left ventricular function was depressed, findings that were corroborated in the present cohort.
Implantation Procedure and Related ComplicationsThe 2-incision surgical technique was used from the sixth patient in the series, with intermuscular placement of the generator in all patients. There was 1 periprocedural hematoma (2%) and 1 infection (2%) during follow-up that did not require surgical revision. In the largest series published on patients with S-ICDs,17 the 30-day rate of device- or procedure-related postimplantation complications was 4.7% in 1637 patients. This rate was 4.5% in the pooled analysis of the IDE and EFFORTLESS studies,24 with a 3-year complication rate of 11.1% in 882 patients; the rate of infection requiring surgical revision was higher (1.7% of patients). The new 2-incision technique simplifies the procedure by avoiding superior parasternal incision, which reduces the procedural time. In this series, with 90% of implantations performed with the 2-incision technique, the mean procedural time was 59.8minutes, whereas in the S-ICD PAS study,17 with only 52.2% of procedures performed using this technique, the time was 77.3minutes. In addition, there are esthetic and comfort benefits for the patient, the risk of infections and erosions is minimized, and it has been shown to be safe, with minimum long-term risk of dislodgments.5,7,8 Moreover, the intermuscular placement of the generator, between the anterior surface of the serratus anterior and the posterior surface of the latissimus dorsi muscles, not only has esthetic advantages, but also reduces pocket complications and IS frequency.8 Additionally, placement of the generator on the muscle without underlying fat tissue would lower the defibrillation threshold.25 In this series, 100% of induced ventricular fibrillations were defibrillated with the device, 95.6% with the first programmed shock (65J). For this reason, there is disagreement about whether to perform induction and defibrillation testing in patients with an S-ICD implant,26,27 particularly those with intermuscular implantation.27
Device TherapiesThe most notable result of the present study is the absence of ISs during follow-up, which may be for multiple reasons.
In the first published experiences with the S-ICD,1 one of the main limitations was a rate of ISs (particularly due to T wave oversensing and supraventricular arrhythmias with heart rates exceeding the programmed detection zone) markedly higher than that observed with transvenous ICD with contemporary programming. A 2-year pooled analysis of the patients in the IDE and EFFORTLESS studies24 revealed that dual-zone programming–1 conditional and 1 shock–instead of a single shock zone was associated with a highly significant reduction in the IS rate. Thus, the dual-zone programming in all patients in the present series has probably contributed to the complete lack of ISs during long-term follow-up.
On the other hand, Olde Nordkamp et al.21 determined an IS incidence of 8.3% in 581 patients after a mean follow-up of 21 months but found that hypertrophic cardiomyopathy and history of atrial fibrillation were independently associated with higher risk of ISs. In the present work, only 1 patient had history of atrial fibrillation and just 18% had hypertrophic cardiomyopathy.
Because our experience with these devices was later than in other countries, most of the patients benefited from device software updates aimed at significantly reducing signal oversensing, and thus ISs. This has partially contributed to the observed rate of ISs and the differences with previous series. T wave oversensing was reduced by an estimated 40% with the introduction of the ACWADD algorithm, with an additional 40% reduction in ISs with the SMART Pass algorithm. The long-term results of ongoing studies that recruited patients at a similar time to our study, such as the S-ICD PAS17 (begun in August 2013), will show the true rate of ISs after the implementation of the device software updates in a larger population of patients.
Only 1 patient received appropriate shocks during follow-up, and the therapies were effective. The effectiveness of S-ICD to terminate spontaneously-induced malignant ventricular arrhythmia episodes was very high and similar to that seen with transvenous ICDs.24
LimitationsThe main limitations of the present work are as follows: a) its inclusion of a low number of patients and those who were not consecutive patients with an ICD indication, which involves a clear selection bias; b) its single-center nature, which might mean that its results are not generalizable to other centers and operators, particularly those with less experience with device implantation; c) its nonrandomized nature, although the aim of the work was not to compare the results with transvenous ICD, but to show our experience with S-ICD after the improvements introduced since its introduction on the market; and d) its relatively short follow-up, although most follow-up durations exceeded 12 months and there were significant differences in the IS rate and complications with surgical revision vs previous S-ICD patient cohorts with similar or even shorter follow-up durations.
CONCLUSIONSImprovements in technology, implantation technique, and device programming, along with appropriate patient selection, have led to outstanding short- and long-term results in this cohort of patients from a contemporary S-ICD implantation center, particularly regarding the absence of both ISs and complications requiring surgical revision.
CONFLICTS OF INTERESTM.A. Arias is a proctor of the Boston Scientific S-ICD system and is an Associate Editor of Revista Española de Cardiología.
- –
Although S-ICD is a therapy with equivalent effectiveness to transvenous ICD that avoids the complications of endovascular leads, it was associated with higher incidence of ISs in the first published series.
- –
Improvements have been implemented in device programming, implantation technique, and software to reduce the incidence of complications. The effects of these improvements are still unknown.
- –
The present work allows an approximation of complications with contemporary S-ICD implantation by including most of the improvements implemented in the therapy since its release on to the market.
- –
In the long-term, with a mean follow-up of almost 18 months, there were no ISs or complications requiring surgical revision, which confirms better results vs those of the initial studies with this therapy.