To the Editor:
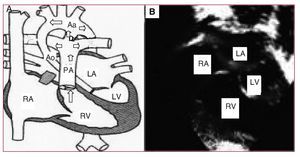
We present the case of a newborn brought to our service with cardiogenic shock. She was diagnosed with hypoplastic left heart syndrome (HLHS) and admitted to the neonatal intensive care unit to initiate treatment with prostaglandins (PGE1), inotropic agents, and support measures. The echocardiographic study showed an extreme HLHS morphology, with mitral-aortic atresia, and very severe ascending aorta hypoplasia (ascending aorta diameter, 2 mm) with retrograde ductal-dependent coronary flow from the aortic arch (Figure 1).
Figure 1. Preoperative status. A: diagram of the pathophysiology of the hypoplastic left heart. The arrows indicate the predominant direction of blood flow, highlighting the passage from the PA through the ductus toward the aortic arch, and in retrograde fashion toward the ascending aorta. B: echocardiographic image of the hypoplastic left heart. Aa indicates aortic arch; Ao, hypoplastic ascending aorta; D, ductus; LA, left atrium; PA, pulmonary artery; RA, right atrium; RV, right ventricle; LV, left ventricle.
Because the results of the Norwood procedure used for initial correction of HLHS are highly influenced by the patient's anatomy and clinical condition,1-2 a decision was made to perform a hybrid technique as an alternative to initial Norwood correction.
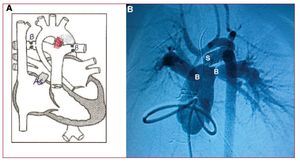
Surgery was performed through a midline sternotomy, in which the typical HLHS anatomy was found: dilated pulmonary trunk, a large systemic ductus, absence of left ventricular chamber, and an extraordinarily hypoplastic ascending aorta (smallest diameter, 2 mm) (Figure 1A). In view of the patient's extreme anatomy and hemodynamic state, we performed a hybrid procedure, inserting a 6-mm diameter,18-mm long coronary stent (LifeStent SDS) in the ductus to keep it open permanently and to ensure systemic blood flow. The stent was placed by purse-string suture at the base of the pulmonary trunk, then deployed and dilated by digital control in its correct location, securing the distal end in the aortic isthmus and the distal proximal end at the origin of the left pulmonary branch. The procedure was completed by bilateral pulmonary artery banding to adequately balance pulmonary flow and systemic flow. Five days later, an atrioseptostomy was performed in the interventional cardiology room to achieve drainage without restriction of the left atrium (Figure 2).
Figure. 2. Post-hybrid treatment status. A: diagram of the hybrid treatment. B: catheterization image 5 days after hybrid treatment, during which atrioseptostomy was performed. As indicates area of percutaneous (Rashkind) atrioseptostomy; B, banding of both pulmonary branches; S, stent placed in the ductus from its origin in the pulmonary artery up to its insertion in the aortic isthmus (shown in red in A).
Postoperative progress was unremarkable under digitalis, diuretic, and antiplatelet therapy. At the time of writing, the patient was receiving follow-up that included echocardiography to assess stent patency while waiting to undergo the future second phase by reconstruction of the ascending aorta and bidirectional Glenn anastomosis.
The mean survival of the Norwood procedure is 60% to 70% in experienced centers,1,2 and closely related to the anatomical shape of the HLHS. In cases with extremely hypoplastic aorta (diameter <2-3 mm) and retrograde coronary flow, the outcome of this procedure is considerably poorer.1-4
The hybrid procedure described here fulfills the objectives of the classic Norwood procedure: it uses a stent to ensure systemic blood flow to keep the ductus open and uses bilateral pulmonary artery banding to control pulmonary pressure and ensure adequate balance between pulmonary and systemic flow. Percutaneous atrial septostomy is always needed to allow nonobstructive drainage of the left atrium over time.
This procedure permits a second phase consisting of reconstruction of the ascending aorta and aortic arch with larger anatomical structures, thus posing a lower risk than the Norwood procedure in newborns with extreme forms of HLHS.
The hybrid surgery does not require extracorporeal circulation or deep hypothermia with circulatory arrest, which facilitates the inevitable repeat surgery in these patients and decreases the risk of neurological lesions.
Our team has decided to use this hybrid technique in extreme forms of HLHS, which, in our experience, show poorer outcome with the Norwood technique. The advantages of the hybrid technique are short procedure time, gradual control of pulmonary flow, and no requirement for extracorporeal circulation. The good initial results obtained by groups in other countries2,5 (this is the first report on a successfully treated patient in Spain) is encouraging for future use of this technique in patients with more extreme forms of HLHS. Nevertheless, larger patient groups are needed to verify the adequate patency of stents placed in the ductus and to assess the morbidity induced by bilateral pulmonary artery banding until the optimal time is reached to complete the second phase of surgical correction among these HLHS patients.