Keywords
INTRODUCTION
The introduction of techniques such as cryopreservation enabled an important advance in the field of tissue preservation.1 The progress achieved gave impetus to the creation of banks of vascular homografts for their eventual utilization in vascular reconstruction.1 Employing cryogenic temperatures to maintain vascular integrity in suspension over prolonged periods of time, the method permits its recovery when the tissue is to be utilized. However, during cryopreservation, changes in the cellular and extracellular components that affect the viscoelastic behavior of the vessel wall have been reported,2-6 and the capability of cryopreservation methods to maintain arterial hemodynamic function is a matter of controversy.
In evaluating cryopreservation methodology and/or the potential clinical utility of a vascular prosthesis, physiological aspects and the experimental design should be taken into account. With respect to the former, it is essential to evaluate the capacity of the prosthesis to develop the functions that it will have to perform after its implantation. Every arterial segment carries out two major, essentially mechanical, functions: to deliver blood to the tissues, imposing low impedance to flow (conduit function), and to mitigate the pulsatility generated by the ejection fraction (arterial wall damping function).2,3 Both functions are determined by the geometric features and the elastic and viscous response of the vessel wall.2,3 To our knowledge, the conduit and damping functions of arterial and venous homografts after cryopreservation have not been assessed to date.
The confirmation of the presence of biomechanical differences related to the cyropreservation procedure would make it possible to validate the cryopreservation technique and/or undertake measures to improve it. For this purpose, the experimental design should take into account the evaluation of the viscoelastic and functional behavior of the segments prior to (fresh vessels) and after (cryopreserved/thawed vessels) cryopreservation. Reports assessing the effects of cryopreservation on vascular biomechanics usually compare the mechanical properties of rings or strips obtained from fresh and cryopreserved vessels when subjected to static trials.5,6 However, only those studies that respect the geometric features and wall structure of the vascular homografts after their implantation yield an adequate functional analysis. On the other hand, for the evaluation of the vascular viscous response and hemodynamic function, it is necessary to analyze the homografts by means of dynamic studies and under hemodynamic conditions that mimic in vivo physiological conditions.
In a number of studies, it has been proposed that the lesser the differences or lack of compliance between the prosthesis and the native artery in terms of viscoelasticity, the lesser the degree of intimal hyperplasia produced in the zone of the anastomosis and, consequently, the lower the incidence of prosthetic failure.7,8 Thus, it has been proposed that the "ideal" graft or prosthesis should reproduce the biomechanical functions of the native vessel.7,8 Only the comparison of the biomechanical and functional properties of vascular homografts with those of the recipient arteries (in vivo) and with those of the most widely utilized prostheses (for example, saphenous veins [SV] and expanded polytetrafluoroethylene [ePTFE]) would make it possible to establish whether homografts reduce the viscoelastic and functional differences observed with respect to native arteries. This evaluation involves in vivo and in vitro studies and requires compatible methodologies that enable the comparison of the results obtained. To the best of our knowledge, this evaluation has not been reported previously.
The objectives of the present study were: a) to determine whether the methods of cryopreservation employed in our tissue bank enable the maintenance of the viscoelastic and functional properties of human muscular arteries and of human veins; and b) to characterize the viscoelasticity and functional capacity of the muscular arteries of potential recipients, comparing them with those of fresh and cryopreserved arterial and venous homografts and those of ePTFE prostheses.
METHODS
The pressure, flow, diameter and vessel wall thickness were determined by in vivo studies using noninvasive techniques and in vitro studies in a mock circulatory system. All the procedures were approved by the ethics committees of the participating institutions.
Noninvasive Recordings
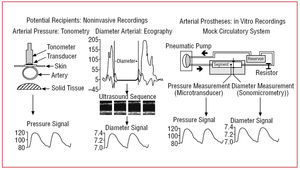
Fifteen normotensive individuals (aged 51±11 years) were studied after remaining in the supine position for 10 minutes. The femoral artery (FA) wall thickness was measured using B mode ultrasound images. The arteries were examined by means of a 7.5-MHz transducer (ATL HDI 5000, Miami Lakes, Florida, United States) until it was possible to visualize 2 parallel lines in posterior wall, corresponding to the lumen-intima and media-adventitia interfaces. When at least 1 cm of the parallel lines was visible, the image at end-diastole was frozen and transferred to a computer by means of a video acquisition tablet. The analysis, based on the gray level intensity and specific tissue recognition algorithms, was performed automatically using software that determined the intima-media thickness.9,10 An image sequence was subsequently recorded and a border recognition algorithm analogous to the intima-media thickness was applied to calculate the diameter curve for an average pulse (Figure 1).9,10 Ultrasound was employed to measure the flow. To record the pressure signal, a pen-like tonometer (Millar Instruments, Inc.) was placed on the skin adjacent to the FA (Figure 1).9,10 To calibrate the signal, the mean and diastolic FA pressures were recorded using a sphygmomanometer at the time of digitization.9,10 These values were assigned to the mean and minimum values of the tonometric signal, respectively. On the basis of the digitized tonometric signal, the values corresponding to each pulse were interpolated and averaged to obtain the pressure curve of an average pulse.9,10
Figure 1. Methods utilized to obtain the pressure and diameter signals.
Concomitantly with the ultrasound and tonometric recordings, a surface electrocardiogram was performed to enable the synchronization of the pressure and diameter signals.9,10
In Vitro Recordings
Segments of FA and SV were obtained from 15 multiorgan donors (aged 30±5 years), selected according to the legal criteria regulating donation (Law 14 005 of the República Oriental del Uruguay). The donor exclusion criteria were in agreement with the norms of the Spanish Association of Tissue Banks. Segments measuring 5 cm in length were cut (warm ischemia for 53 to 60 minutes) from each FA and SV (left and right). They were washed and stored in a physiological solution at 4°C for 24 to 48 hours (cold ischemia). We studied a fresh specimen of FA and SV from each donor and the remaining specimens were studied after cryopreservation. The method employed is similar to that utilized previously (Table 1).11
The vascular segments and 15 segments of ePTFE (6 mm, Gore-Tex Vascular Graft, W.L. Gore & Associates, Inc., Flagstaff, Arizona, United States) were studied in a mock circulatory system (Figure 1).10-12 The segments were immersed in and perfused with Tyrode solution (oxygenated, 37°C, pH 7.4). Each segment was instrumented with a pressure transducer (Konigsberg Instruments, Inc., Pasadena, California, United States) and a couple of ultrasonic crystals adapted for sonomicrometric recording (Triton Technology, San Diego, California, United States).10-12 Once instrumented, the segments were subjected to waveforms, frequencies, pressures and flows similar to those recorded in normotensive individuals.10-12 Thirty pulses were digitized for each segment (sampling frequency, 200 Hz). A detailed description of the mock circulatory system and the experimental methodology is provided in previous publications.3,9,12
Data Analysis
Elastic and Viscous Response
The representation of the vessel wall using a Kelvin-Voigt viscoelastic model (Figure 2) permitted the calculation of the pressure-diameter elastic (Epd) and viscous (Vpd) moduli.3,9,12 According to this model, the total pressure exerted on the wall consists of separate elements representing the elasticity or elastic component (spring) and the viscosity or viscous component (damper) (Figure 2):
Figure 2. Diagram of the Kelvin-Voigt model. Epd indicates pressure-diameter elastic modulus; Vpd, pressure-diameter viscous modulus.
A rearrangement of the equation results in:
The Pviscous is proportional to the first derivative of the diameter over time:
where Vpd is the viscous modulus and dD/dt, the first derivative of the diameter over time. On the basis of the pressure and diameter signals, we constructed the pressure-diameter relationship for each pulse analyzed.3,9,12 The area of hysteresis of the relationship was reduced by iteratively incrementing the Vpd, which was achieved with software especially designed for this purpose.3,9,12 Once the minimum area had been obtained, the incremental iterative procedure was discontinued and the Vpd obtained was considered to be equivalent to the arterial wall viscous modulus.3,9,12
The following exponential function was fitted to the pressure-diameter relationship obtained upon elimination of the area of hysteresis:
The Epd was calculated as the slope of the function at the mean diastolic pressure3,9,12:
Wall Damping and Conduit Function
Using a Kelvin-Voigt model, the capacity of the arterial wall to damp a pressure stimulus is obtained from the relationship between the pressure stimulus and the resulting strain. As in previous studies, the wall damping function (WDF) was quantified as:
The lower the WDF, the lower the damping capacity of the arterial wall.3,9,12
The conduit function (CF) was calculated as 1/Zc, where Zc is the characteristic impedance of the segment, calculated as2:
where PWV is the pulse wave velocity, ρs is the density of the perfusion fluid and ATS is the area of the transverse section.2
Elastic Response: Parameters Independent of the Diameter
In order to determine arterial stiffness independent of arterial dimensions, we calculated the incremental Young's modulus (EINC) and the clinical indicators PWV and the pressure-strain elastic modulus (E¿).2,3,9,12 To determine the EINC, we calculated the vascular circumferential stress (σ) and vascular strain (ε), according to the following equations:
where P is the pressure, Ri and Re are the arterial internal and external radius, respectively, R is the mean radius (R=[Ri+Re]/2), and RO is the arterial radius at a pressure of 0 mm Hg. The stress-strain relationship was constructed for each pulse. Using a procedure similar to that described above, the area of hysteresis was eliminated and a pure elastic stress-strain relationship was obtained.13 The EINC was calculated as:
where dσ and dε are the first derivatives of stress and strain, respectively, with respect to time.2,3,9,12 The EINC was calculated for a dσ/dε corresponding to the mean diastolic stress.3,9,12
The pulse wave velocity was calculated as:
where Hm is the mean arterial wall thickness and ρ is the blood density (ρ=1.06 g/mL).2,3,9,12
The EP was calculated as2,3,9,12:
where PS and PD are systolic pressure and diastolic pressure, respectively, and DS and DD are the systolic diameter and diastolic diameter, respectively.
Viscoelastic and Functional Noncompliance
The lack of viscoelastic and functional compliance between the FA of potential recipients and the vascular prostheses was calculated as follows:
We quantified the noncompliance for each parameter assessed. For the calculation and paired comparison of these parameters, a homograft or ePTFE segment was randomly assigned to each FA of the potential recipients. When the above equation was employed, the values corresponding to the lack of compliance ranged between -1 and 1. Values close to 0 indicate a lower level of noncompliance and values close to -1 or 1 indicate maximum noncompliance.
Statistical Analysis
The values calculated for each segment were averaged and expressed as the mean value plus or minus the standard deviation (SD). Multiple paired comparisons between all the groups considered were carried out in every case using analysis of variance (ANOVA), followed by the Bonferroni test. P< /I><.05 was considered to indicate statistical significance.
RESULTS
Table 2 shows the hemodynamic variables. The similarities in the values for pressure, flow and frequency made it possible to compare the parameters in Tables 3, 4, and 5 in isobaric, isoflow and isofrequency terms. Of all the segments studied, only the ePTFE prostheses presented significant differences in diameter (P<.05).
The biomechanical parameters Epd and Vpd and the WDF and CF for each group are shown in Table 3. There were no significant differences between fresh and cryopreserved segments of the arterial and venous homografts. All the prostheses presented differences with respect to the FA of potential recipients in terms of Epd, Vpd CF, and WDF (P<.05). There were no significant differences in Epd or CF when arterial and venous homografts were compared (P>.05). The viscous response and WDF of the arterial homografts differed from those of SV and ePTFE (P<.05).
Table 4 shows the values for PWV, EINC and E. There were no differences between fresh and cryopreserved segments of the arterial and venous homografts (P>.05). The evaluation of the EINC, Ep and PWV demonstrated that the arteries of the recipients were less stiff than the homografts and ePTFE. Only the Ep presented significant differences when the arterial and venous homografts were compared (P<.05).
The values representing the lack of compliance between the FA of patients and the vascular prostheses appear in Table 5. The values corresponding to fresh and cryopreserved homografts were similar in the case of both arteries and veins. The most marked noncompliance was observed in ePTFE, regardless of the parameter being considered. In the comparison between native FA and the homografts, the compliance was greatest for arterial homografts when Vpd and Ep were considered.
DISCUSSION
The following discussion focuses on the 2 major findings of this study:
1. Cryopreservation preserved the elastic and viscous responses as well as the hemodynamic function of the arterial and venous homografts.
2. Fresh and cryopreserved arterial homografts were those showing the greatest compliance with the FA of patients in viscoelastic and functional terms.
Elastic and Viscous Response
The vascular elastic response determines vascular function. An adequate elastic modulus permits systolic distension and subsequent diastolic elastic recoil, which ensures the continuity of antegrade blood flow2,3 and reduces the oscillations generated by the heart, ventricular afterload and the probability of vascular rupture due to overdistension.2,3
To evaluate the elastic response, we utilized different indicators of elasticity that provide complementary information.3,12 The EINC is considered to be the reference standard for the evaluation of the elastic response of a material as it is the index that best defines (independently of the size or geometry) the intrinsic properties of the material being studied.2,3,12 Given that it is difficult to calculate (it is necessary to know the thickness, diameter and instantaneous strain of the vessel), this modulus is seldom used in the clinical setting.2,3,12 Another of the indicators studied was the PWV, which enables the evaluation of the capacity of the vessel to propagate pulse waves along its walls. Like the PWV, the other indicators utilized, the Epd and the Ep, can easily be calculated in the clinical setting since they only require the measurement of the arterial pressure and diameter. The differences between the Epd and the Ep lie in that the calculation of the former, like that of the EINC and the PWV, requires the definition of the pressure value for which it is being calculated and that it enables the evaluation of vascular stiffness independently of the viscous properties of the wall given that, for this calculation, the hysteresis of the pressure-diameter relationship is eliminated.12 In this regard, it should be pointed out that the method utilized for the calculation of the Epd employs mainly the pressure-diameter relationship at end diastole, where the only elastic phenomena are due to the relaxation of the arterial wall. Moreover, the Epd makes it possible to calculate the WDF as it involves units consistent with the Vpd.12 On the other hand, the Ep enables the evaluation of arterial stiffness in relation to the unit strain and, as such, is independent of the diameter.3,12 Its clinical utility lies in the fact that it is only necessary to know the maximum systolic and minimum diastolic values of the arterial pressure and diameter signals in order to perform the calculation.3,12 However, since the determination requires the use of these maximum and minimum values, it contains certain spurious elements associated with the systolic peak of the pressure-diameter relationship. These elements include the pulse wave reflection, which affects the zone near the maximum systolic point, and elements that may be determinants of the area of hysteresis, which include the vessel wall viscosity.2,12
Our findings show that, regardless of the parameter utilized to evaluate the elastic response (EINC, PWV, Epd, or Ep), cryopreservation did not lead to differences between fresh and cryopreserved arterial and venous homografts.
Concerning the compliance between the segments studied and the native AF, the results vary depending on the parameter employed. When the parameters analyzed measured the elastic response independently of the viscous response (EINC, PWV, and Epd), the arterial and venous homografts presented differences with respect to the native arteries, but there were no significant differences between the two types of homografts. Consequently, the implantation of a fresh or cryopreserved arterial of venous homograft would generate, at least initially, similar values in terms of "elastic noncompliance" with respect to the recipient FA.
The viscous response of the vessel wall causes the arterial system in systole to dissipate part of the energy imparted by the heart in each pulse in the form of heat.3,10,12
The viscosity attenuates the highest frequency components of the incident waves of pressure and flow and the amplitude of the reflected waves, which could trigger resonance phenomena in the system and damage the arterial wall.3,10,12
The results demonstrated that there were no differences in the viscosity (Vpd) when fresh and cryopreserved arterial and venous homografts were compared. Thus, the cryopreservation method not only conserved the elastic response, but the viscous response as well. On the other hand, the Vpd of the arterial homografts was that most similar to that of recipient FA.
Finally, it should be pointed out that, regardless of the parameter assessed (Tables 3 and 4), the ePTFE segments presented the greatest differences with respect to the FA of potential recipients.
Our findings indicate that arterial homografts, fresh or cryopreserved, can be considered interesting alternatives as muscular artery prostheses that minimize the differences in viscoelasticity with respect to native arteries.
Vascular Function
The cryopreservation procedure did not affect the WDF of the muscular arteries or the veins. Moreover, the results obtained show that the arterial homografts presented the greatest compliance with the FA of potential recipients in terms of the damping capacity, followed by the venous homografts. A lower capacity to damp the pulsatility and, consequently, to protect itself from this action may be related to vessel wall hyperplastic responses (adaptive responses), which are observed in venous prostheses implanted in arterial territory.
Considering the results obtained, at least from a theoretical point of view, fresh and cryopreserved arterial homografts would constitute vascular prostheses that would be more effective than other types of prostheses in damping the pulsatility of the pressure and flow waves. This would result in less pulsatile pressure and flow and, thus, reduced oscillations in circumferential and shear stress in distal arteries (anastomotic and postanastomotic). The importance of these factors is evident if we take into account the fact that marked oscillations in pressure or in vessel wall circumferential and shear stress have been associated with wall damage and the resulting vascular hyperplastic response.7,8
The conduit function was quantified as the inverse of the characteristic impedance (Zc).3 The importance of knowing the impedance to flow imposed by the native arteries and vascular prostheses was reported in earlier studies that demonstrated that hemodynamic disturbances occur at those sites subjected to sudden changes or to noncompliance in terms of impedance. These disturbances compromise the segments involved, as well as the rest of the cardiovascular system.7,8 The greater the lack of compliance in impedances or in the conduction caprosthesis, the less successful the reestablishment of the blood flow, the higher the probability of thrombotic events and/or hyperplasia and, if implanted in large vessels, the greater the ventricular afterload.7,8,13
Cryopreservation enabled the maintenance of the conduit function of arterial and venous homografts, although, when compared with the FA of patients, differences were detected in all the prostheses. However, there were no differences between the venous and arterial homografts, while the segments of ePTFE presented the most marked differences with respect to native arteries.
The results obtained suggest that the interposition of an arterial and/or venous homograft in arterial territory would generate a similar degree of impedance to flow and lack of compliance with anastomotic impedances. Likewise, the degree of noncompliance in terms of impedance would always be lower than that resulting from the interposition of an ePTFE prosthesis.
Clinical Implications
Cryopreserved segments, venous and/or arterial, are utilized in a number of clinical situations: revascularization surgery, vascular access for hemodialysis, reconstruction of the cavities of the heart, etc.14,15 However, the capacity of cryopreservation to maintain the viscoelastic and functional properties of the homograft and, thus, the capacity of the vessel walls to adapt to the hemodynamic conditions of the recipient, is a matter of controversy.
The hemodynamic status of recipients of arterial homografts can vary widely, and a number of vascular diseases can be involved. In this respect, given that certain situations can change the mechanical properties of the vessels, it would be of interest to carry out the biomechanical evaluation of the native vessels of the recipient in order to select the most suitable vascular prosthesis.
The results of this study show that the method of cryopreservation employed preserves the elastic and viscous responses, as well as the hemodynamic functions, of arterial and venous homografts. Consequently, this methodology would make it possible to store cryopreserved vascular homografts in tissue banks, where viscoelastic and functional properties similar to those of fresh segments would be maintained.
However, the results demonstrate that the fact that the homografts continue to show the properties of fresh segments does not mean that they present properties similar to the arterial segments in vivo. In this regard, in the present study, the fresh segments exhibited a greater elastic response and a reduced viscous response when compared with the arteries of potential recipients. This circumstance could be related to the loss of the regulation of smooth muscle tone in the segments studied in vitro. In this respect, in recent studies, we have shown that the baseline smooth muscle tone makes it possible to maintain a lower elasticity and a higher viscosity in the vessel and, thus, maintain an elevated damping capacity.10 Therefore, the major strategy for conserving the degree of vascular viscoelasticity and functionality found in vivo may be to minimize the changes in the wall produced in homografts prior to cryopreservation.
Our work also demonstrates the need to employ parameters that measure not only the elastic response, but the viscous response as well, through the analysis of the lack of compliance between vascular prosthese and native arteries. Otherwise, the conclusions reached concerning the degree of compliance between native vessels and vascular prostheses may be partial or erroneous.
We wish to stress the fact that a proper assessment of the clinical utility of these homografts requires additional in vitro studies to evaluate other variables (for example, muscle reactivity and endothelial function), as well as a phase during which in vivo studies would be performed to evaluate the postimplantation behavior of the prosthesis. In any case, preimplantation studies of this type make it possible to carry out an objective analysis of the "expectable" degree of viscoelastic and functional compliance in vivo, independently of the factors that might influence the viscoelasticity of the homograft once implanted.
Finally, this report presents a noninvasive method for recording wall pressure, diameter and thickness signals that enables a detailed biomechanical and functional analysis of human arteries. This methodology could be of considerable utility in the postimplantation biomechanical and functional evaluation of homografts and native arteries.
CONCLUSIONS
The cryopreservation procedure employed preserved the degree of elastic and viscous response, as well as the conduction and damping capacity of arterial and venous homografts.
All the vascular prostheses presented differences with respect to the arteries of potential recipients. Arterial homografts showed the greatest viscoelastic and functional compliance with the native arteries of patients, followed by venous homografts.
ACKNOWLEDGMENTS
The authors wish to thank Elbio Agote and the staff of the Instituto Nacional de Donación y Trasplante de Células, Tejidos y Órganos (National Institute for Donation and Transplantation of Cells, Tissues, and Organs, INDT), Rosario García (Buquebus) and the Spanish Society of Cardiology for the award granted to this work at the Congress of Cardiovascular Diseases (Barcelona, Spain, October 2005).
Correspondence: Dr. D. Bia.
Facultad de Medicina. Departamento de Fisiología. Universidad de la República.
General Flores, 2125. 11800 Montevideo. Uruguay.
E-mail: dbia@fmed.edu.uy
Received December 27, 2005.
Accepted for publication March 28, 2006.