.
The European Congress of Cardiology witnessed during only a 5-year time period the most opposite perceptions medical progress can engender, both excitement and disappointment. Drug-eluting stents (DES) were responsible for that. In Stockholm 2001 the first DES-trial (RAVEL: randomized study with the sirolimus-coated BX™ velocity balloon-expandable stent) yielded awesome results for sirolimus-eluting stents but only 5 years later, in Barcelona 2006, some meta-analyses linked the use of DES to a higher risk for long-term mortality. Very late stent thrombosis, which until then had only been the subject of scarce case reports turned into “the great threat after DES implantation.”.
From this moment on the interventional cardiologist community undertook tremendous efforts to elucidate the underlying truth. Newer, detailed, patient-level meta-analyses were published, as well as numerous and ample registries. Moreover, consensus definitions of stent thrombosis, including definite, probable, and possible stent thrombosis, were created to better define the issue.1.
Regarding thrombosis and particularly very late thrombosis, a study large enough to assess this problem conclusively would have to include more than 10 000 patients. There are no such studies. Therefore, high-quality meta-analyses, if possible at patient level, were in order to resolve the controversy regarding the use of DES..
It is also important to acknowledge that patients included in early trials are carefully selected, with a risk profile lower than the patient population encountered in clinical practice. In response to this, some “all comers” trials have been designed with minimal exclusion criteria in order to enrich higher-risk patient and lesion populations. Registries entail several limitations, particularly the problem of bias due to known and unknown confounding factors, which are frequently not resolved even after adjustment or propensity score matched analysis..
Drug eluting stents: class effect for very late stent thrombosis?To suggest a class effect of various DES regarding the problem of very late stent thrombosis is somewhat naïve, bearing in mind the complexity of these devices that are composed of:
• Metallic platform (material, geometry, filament thickness)..
• Polymer (composition, disposal, thickness, biocompatibility, thrombogenicity, pro-inflammatory potential, biodegradability or even the lack of polymer itself)..
• Antiproliferate drug (molecular composition, biologic actions, doses, releasing kinetics)..
Initial trials with first-generation DES, sirolimus-eluting (SES), and paclitaxel-eluting stents (PES) did not point to an increase in early stent thrombosis.2 Late and very late stent thrombosis cases were described in some case reports. However, large scale registries of the unrestricted use of DES observed an annual risk of very late stent thrombosis after the first year of 0.4% to 0.6%, which was a disturbing matter of concern.3, 4.
Several meta-analyses comparing first-generation DES with bare-metal stents were performed, with follow-up up to 4 years and using both the Academic Research Consortium stent thrombosis definitions as well as the original per-protocol definitions. These showed a similar definite and probable stent thrombosis rate.5 However, a significant increment in very late stent thrombosis for DES was found when using per-protocol definitions..
The apparent contradiction between registries and randomized trials might be explained by the lower risk profile of patients included in these trials. The “off label” clinical and angiographic characteristics have often been pointed to as thrombosis predictors..
Regarding the impact of DES on the risk of death and myocardial infarction, none of the meta-analyses has shown a difference between DES and bare-metal stents.6 One explanation for the lack of increased risk of death or myocardial infarction with DES, despite a certain propensity for very late stent thrombosis, is likely related to the profound reduction in the risk of restenosis and repeat revascularization, and potentially a lower risk of early stent thrombosis..
In addition, ST segment elevation myocardial infarction has to be taken into account as a particular clinical setting. Although a large meta-analysis did not find a greater rate of thrombosis, death, or infarction in up to 2 years of follow-up,7 there remains concern about an increased risk of very late stent thrombosis.8.
It can therefore be concluded that first-generation DES are associated with a greater risk of very late stent thrombosis, that the event rate is relatively low, and that the adverse event is more prevalent in higher-risk patients and lesions without translating into an increased risk of death or myocardial infarction..
The second drug-eluting stents generation Everolimus-Eluting Stent: Comparative Trials With First-Generation Drug-Eluting StentsComparative studies vs PES (SPIRIT II, III, IV and COMPARE) have consistently shown a lower stent thrombosis rate using the everolimus-eluting stent (EES). The difference was significant in the 2 largest trials (SPIRIT IV and COMPARE) and pooled analyses have shown a significantly lower risk of definite and probable stent thrombosis during a mean follow-up of more than 2 years (hazard ratio [HR]=0.35; 95% confidence interval [CI95%], 0.21-0.6).9, 10.
The combined analysis of all SPIRIT studies showed an annual risk of definite and probable stent thrombosis after the first year and up to the third year of 0.17% with EES and 0.37% with PES.11 At 3 years, the incidence of stent thrombosis with EES is half the incidence with PES (0.74% vs 1.65%; P=.003). A study with minimal exclusion criteria, COMPARE, showed an annual risk of definite and probable stent thrombosis after the first and up to the third year of 0.35% with EES and 1.15% with PES. At 3 years the incidence with EES was 1.4% compared to 4.9% with PES (P<.0001).11.
Comparison of second-generation DES vs SES is more challenging. Studies in general show a smaller number of thrombosis events with EES. In the main trials (SORT OUT IV, EXCELLENT, and ISAR TEST 4) a trend for lower thrombosis rate is observed with EES.12 The meta-analysis showed a trend towards fewer definite and probable stent thrombosis with EES (HR=0.78; 95%CI, 0.52-1.18),12 a significantly lower risk of definite stent thrombosis with EES during the first year (HR=0.29; 95%CI, 0.13-0.66), and a trend towards a lower risk of very late definite thrombosis (HR=0.34; 95%CI, 0.09-1.22) (Palmerini et al., presented at Transcatheter Therapeutics Meeting, 2011, San Francisco, California, United States)..
It is important to note that the weighted follow up of these studies is shorter than those with PES. The 2 largest studies have a follow up of 9 and 12 months as opposed to 24 months in studies with PES..
Everolimus-Eluting Stent: RegistriesThe LESSON registry compared 2 groups of 1342 patients matched by propensity score with a mean follow up of 18 months. Definite and probable rates of stent thrombosis were lower with EES than SES (2.5% vs 4%; P=.04).13.
The Bern-Rotterdam registry evaluated the rate of stent thrombosis in more than 12 000 patients treated with either PES, SES, or EES. Very late thrombosis risk was reduced in the EES group (76% lower than PES and 67% lower than SES). The annual definite and probable rate of very late stent thrombosis was 0.5% for EES, 1.1% for SES, and 1.4% for PES (Räber et al., presented at the European Society of Cardiology Meeting, 2011, Paris, France)..
The nation-wide SCAAR registry and also the Spanish ESTROFA-2 registry find lower rates of stent thrombosis with second-generation DES when compared to the first generation, with a reduction of 40% to 50% in thrombosis risk.14, 15 The annual rate of definite thrombosis after the first year is 0.2%.
The combined analysis of SPIRIT II, III, and IV trials and the SPIRIT V, SPIRIT woman, XIENCE V USA, and XIENCE V India registries with more than 13 000 patients shows a definite plus probable thrombosis rate at 1 year of 0.61% with only a 0.11% increase in the second year.16.
ST Elevation Myocardial InfarctionIn the EXAMINATION trial the definite and probable stent thrombosis rate at 1 year was significantly lower with EES than with bare metal stents (0.9% vs 2.6%, P=.01).17.
Zotarolimus-Eluting Stents: Comparative Trials With First-Generation Drug-Eluting StentsThe first version of the zotarolimus-eluting stent (ZES) has shown a lower definite plus probable very late thrombosis rate as compared to first-generation DES, both PES (ENDEAVOR IV: 0.2% vs 1.5% after 36 months) and SES (ENDEAVOR III: 0.3% vs 0.9% after 60 months and SORT OUT III: 0% vs 0.2% after 18 months).18, 19, 20.
The new version of ZES, RESOLUTE (ZESr), uses a proprietary new biocompatible polymer blend which confers a slower drug release and allows a lower lumen loss. This stent has been compared to EES in 2 studies. In the RESOLUTE All Comers study, definite plus probable thrombosis rate at 2 years was similar (ZESr 1.9% vs EES 1%). The definite plus probable thrombosis rate in the second year was only 0.3% in both groups.21 The TWENTE study, not yet published, also revealed a comparable definite plus probable thrombosis rate at 1 year (ZESr 0.86% vs EES 0.16%) (Von Birgelen et al., presented at Transcatheter Therapeutics, 2011, San Francisco, California, United States)..
Zotarolimus-Eluting Stents: RegistriesAs far as registries are concerned, the ESTROFA-2 with ZES and the RESOLUTE US with ZESr have shown a very low late thrombosis rate.15, 22 The annual increment in thrombosis after the first year remains ≤0.2%..
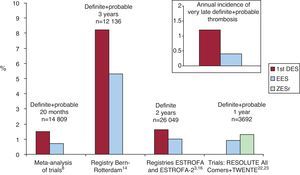
Summary of Comparison Between Drug-Eluting Stents GenerationsComparative trials do not have a suitable design powerful enough to address the thrombosis issue. Nevertheless, these studies do find differences that favor the new generation DES, particularly when compared to PES (Figure 1)..
Figure 1. Comparative thrombosis rates with first- and second-generation drug-eluting stents. 1st DES, first generation drug-eluting stents; EES, everolimus-eluting stents; ZESr, RESOLUTE zotarolimus-eluting stents.
Real clinical practice registries, with a higher-risk population, do consistently show lower thrombotic events with the new DES, with a reduction up to 40% to 70% when compared to first-generation DES (greater with PES) (Figure 1)..
This means that of 1000 patients treated with the new-generation DES in actual practice, after the first year only 3-4 patients/year will suffer from a definite or probable thrombosis. This figure must be put in perspective. Had those 1000 patients been treated with bare-metal stents, 100 to 120 new revascularizations due to restenosis would have been performed, leading to death or infarction in 3 or 5 cases, respectively, not to forget that we should add 3-4 patients/year with very late definite or probable thrombosis in bare-metal stent cases..
When talking about PES it was concluded that thrombosis-related events were balanced with a decrease in restenosis. Now, with the new-generation DES, we do have to conclude that with the lower thrombosis and restenosis risks the balance clearly favors these new stents. Another issue would be to define the most cost-effective scenarios..
Why do second-generation drug-eluting stents portend a lower thrombotic risk?This better performance of EES could be explained in several ways:23
• Metallic platform with much thinner struts compared to the first generation allowing a quicker and more complete endothelization..
• New polymers are very biocompatible with lower thrombogenicity, lower inflammation, and a more attenuated platelet activation..
• The different cellular and molecular actions of the macrocyclic lactone group (“-limus drugs”) and paclitaxel, as well as the different release kinetics, might play a role..
A recent meta-analysis including 8 comparative studies of DES with durable polymers reveals a lower late and very late thrombosis rate with biodegradable polymeric DES (HR=0.6; 95%CI, 0.39-0.91).24.
Fully erodable stents: the fourth generationIt is still too early to know whether these stents will be able to eradicate late thrombosis. The Bioabsorb study presents a follow-up of 4 years with no thrombosis, but only 30 patients were enrolled so any conclusion might appear premature. Nevertheless, follow-up studies with optical coherence tomography show promising findings..
Late thrombosis has changed from a relatively infrequent dreadful complication into a rare event. Given the new designs, in a not very distant future it will remain a topic for isolated case reports..
Prejudices are not easy to eradicate neither in daily life nor in science. Not even the new evidences that dismantle the previous erroneous concepts are always able to reverse their negative impact. The flood of new data and the speed with which they appear do contribute to it. That is the reason why it takes effort to remain open to novel insights, and constantly update evidence in an analytical spirit..
Conflicts of interestJ.M. de la Torre Hernández: testimony, consultancy, grants and payments for lectures (Abbott, Boston Sci, Cordis, IHT, Biotronik, Medtronic, Volcano). Stephan Windecker: grants and payments for lectures (Abbott, Boston Scientific, Biosensors, Cordis, Medtronic)..
Corresponding author: Unidad de Hemodinámica y Cardiología Intervencionista, Hospital Universitario Marques de Valdecilla, Valdecilla Sur, 39012, Santander, Spain. he1thj@humv.es