Keywords
INTRODUCTION
Radiofrequency ablation (RA) is a highly effective treatment in most arrhythmic substrates, with success rates of around 90%.1 However, in certain tachyarrhythmias such as atrial fibrillation, some types of atypical atrial flutter and ventricular tachycardia (VT) in structural heart disease, it is notably less effective, with success rates below 80%.1 In these disorders, effectiveness is lowered mainly because the arrhythmic substrate is less well defined compared with other tachycardias, so the reentrant circuit is harder to locate, particularly in slow conduction zones. In recent years, different nonfluoroscopic techniques for three-dimensional display of the RA catheter have been developed to overcome this difficulty and assist in mapping of complex arrhythmogenic substrates.2-5 One of these, the LocaLisa system, is among the cheapest and least time consuming for implementation and development. Although its usefulness in RA of different tachyarrhythmias has been demonstrated,6-9 RA of VT with this system has not been reported in the scientific literature.
The objective of this study is to describe the usefulness and the results of RA of VT guided by the LocaLisa system in structural heart disease.
METHOD
Patients and Study Design
The present, essentially descriptive, study included 32 consecutive patients with proven sustained monomorphic VT and structural heart disease who underwent electrophysiological study and RA of the arrhythmogenic substrate in our center. The nonfluoroscopic, three-dimensional, real-time, navigation system, LocaLisa, was used, in addition to the conventional fluoroscopic method, to map the catheters during the procedure in 10 patients. The results from this group were compared with those of a control group of 22 patients who underwent the procedure with fluoroscopy only. The last 6 patients in the LocaLisa group were prospectively and consecutively enrolled (8 procedures). The other 4 patients (4 procedures) were retrospectively included. The decision to use the LocaLisa system instead of simple fluoroscopy in these 4 patients was made by the specialist in accordance with the clinical situation of the patient and the complexity of the procedure, the logistic limitations at the time, and the availability of staff with sufficient experience and knowledge of using the system.
The main reason for using the LocaLisa system was not to reduce the fluoroscopy time but rather to accurately locate and define the arrhythmic substrate.
Electrophysiological Substrate
Informed consent was obtained from the patients prior to the procedure. All patients underwent the procedure after fasting, and antiarrhythmic medication had been suspended at least 5 half lives of elimination earlier. Two 5-Fr tetrapolar catheters (Bard Electrophysiology Division, MA, USA) were implanted initially in the right atrium and region of the His bundle, and subsequently at the apex of the right ventricular outflow tract, with transfemoral vein approach. In the LocaLisa group, a 6-Fr active fixation bipolar catheter was also implanted in the right ventricular apex as a reference for the system. The last 7 patients of this group also had a 6-Fr decapolar catheter (Bard Electrophysiology Division, MA, USA) implanted in the coronary sinus as an anatomical reference for localization of the atrioventricular annulus and the base of the left ventricle. In both groups, left ventricular mapping and RA were carried out by deflectable 7-Fr tetrapolar catheters with 4 mm, 8 mm, or irrigated tip distal electrode (Marinr, Conductr, or Sprinkl, respectively, Medtronic, Minneapolis, MN, USA), introduced with retrograde transaortic approach.
All patients received heparin intravenously as a 50 U/kg bolus followed by 1000 U for every hour the electrode remained in the left ventricle.
Poor hemodynamic tolerance of VT was considered as systolic blood pressure below 90 mm Hg in the presence of symptoms of low cardiac output during VT.
Mapping the Circuit
The slow conduction zone of the circuit was identified by conventional mapping techniques. Initially, in patients without incessant VT, activation was mapped in sinus rhythm or during pacing from the right ventricular apex in search of fragmented low amplitude potentials in the latest portions of the QRS complex.10 Pacing of the suspected tachycardia site was also used to search for S-QRS durations longer than 60 ms during sinus rhythm with VT morphology in at least 11 of the 12 electrocardiographic leads. We then attempted to induce clinical VT to identify the slow conduction zone of the tachycardia circuit with the following criteria: presystolic and mid-diastolic potentials, demonstration of entrainment with concealed fusion, postentrainment cycles up to 30 ms longer than the VT cycle and electrogram to QRS interval during VT equal to the spike-QRS interval during entrainment.11-13
Nonfluoroscopic navigation With the LocaLisa System
The technical details and operation of the LocaLisa system have been described previously.4,14,15 The display is in 3 dimensions and real time. The system can identify and memorize anatomical points corresponding to each electrode position during the procedure, with an error in precision, with respect to the true catheter position, of just 1-2 mm.4,15
For mapping of the VT reentrant circuit, first the RA catheter was guided to the left ventricular apex with the LocaLisa system. Once in position, a point was marked in the system to provide an easy reference between the apex of the ventricle and its base. The base position was established by catheter electrodes placed in the coronary sinus. Then, in sinus rhythm, the points corresponding to scar tissue or to the limits of viable myocardium were marked. All points with a voltage amplitude less than 0.1 mV in the local electrogram were considered scarred.16,17 The extent and location of scar tissue were easily displayed by the LocaLisa system in all spatial projections by rotating the images. After locating the most probable site of the circuit, we attempted to induce VT and work with a two-dimensional display of this circuit, looking for the projection of the LocaLisa system that enabled best visualization. At the same time, fluoroscopy in the projection orthogonal to the LocaLisa system was used to control the displacement of the probe catheter along the axis perpendicular to the chosen projection for the system. During mapping of pacing and entrainment, different points of the scanned region were marked with a color code to indicate whether they belonged to the reentrant circuit or not. The aim was to locate and mark in the system the entry and exit of the slow conduction region.
Radiofrequency Ablation
A programmable radiofrequency generator with continuous monitoring of impedance, voltage and temperature (ATAKR 2, Medtronic, Minneapolis, MN, USA) was used for ablation. Radiofrequency was applied during reproducible and hemodynamically tolerated monomorphic VT to sites that met at least 2 of the aforementioned mapping criteria. Occasionally, in patients who tolerated induced VT poorly, guided radiofrequency applications were made through an unconventional approach by looking for late electrograms during sinus rhythm or pacing from the right ventricular apex.16
All points where radiofrequency was applied were marked in the LocaLisa system, distinguishing between successful and ineffective applications. Radiofrequency ablation was considered successful when clinical VT was terminated and subsequent reinduction was impossible. When RA was applied with no VT because of intolerance, only the second of these conditions had to be met.
Statistical Analysis
Data were expressed as mean ± standard deviation. The Student t test was used for comparison of quantitative variables between the 2 groups. Comparison of qualitative variables between the 2 groups was performed by the χ² test and the Fisher test. Statistical significance was set at P<.05.
RESULTS
Clinical Characteristics
Nine of the patients in the group of patients who underwent RA guided by the LocaLisa system had previous myocardial infarction; the remaining patients had arrhythmogenic right ventricular dysplasia. The control group included 22 patients, all with a history of acute myocardial infarction. The clinical characteristics of these patients are presented in Table 1. No significant differences were seen between the 2 groups for either clinical characteristics or the main RA variables analyzed (Tables 1 and 2).
Mapping and Ablation
Twelve procedures were performed in the LocaLisa group, of which 9 (75%) were successful. This success rate was slightly higher than that obtained in the control group (68%), though the difference was not statistically significant (Table 2). Two patients in the LocaLisa group and 3 in the control group required a second procedure for RA of the arrhythmogenic substrate. Thus, 7 out of 10 (70%) of the patients in the LocaLisa group were successfully treated with RA, whereas the success rate in the control group was 63% (14/22). In 8 patients (8 procedures), clinical tachycardia was similar to the tachycardia mapped during the procedure. As shown in Table 2, there were no significant differences between the 2 groups in the number of VTs induced, mean VT cycle length, mean duration of the procedure, RA or fluoroscopy, or the number of radiofrequency applications. On the other hand, 76% of VTs mapped in the control group showed sufficient hemodynamic tolerance compared to 58% of VTs in the LocaLisa group, but these differences were not significant. One procedure in the control group (4%) required mapping of the arrhythmic substrate during sinus rhythm because of intolerance of VT compared to 4 procedures (33%) in the LocaLisa group (P=.03).
In 1 patient, radiofrequency was successfully applied to the region between exit and entry of the slow conduction zone of the circuit with guidance almost exclusively by the location of these points in the LocaLisa system, given that no mid-diastolic electrical activity was recorded in this zone (Figure 1). Finally, none of the patients in the LocaLisa group discontinued the procedure and no 3-dimensional displays had to be reinitialized due to movement of the reference catheter.
Fig. 1. Ablation of ventricular tachycardia after myocardial infarction, guided by the LocaLisa system. A: image of LocaLisa system in anterior oblique cranial projection. A decapolar catheter is depicted in the position close to the coronary sinus and the approximate outline of the left ventricle is traced with a white line. Grey points represent scars, whereas violet and red points indicate positions of interest in mapping or for ablation, respectively. B and D: electrograms recorded in ablation channel of the two ends of the slow conduction zone of the tachycardia circuit. The upper arrow indicates the point where activation mapping records an electrogram-QRS delay of 400 ms (entry point), whereas the lower arrow indicates where a delay of 40 ms is recorded (exit point). C: trace at the exact moment of termination of tachycardia during application of radiofrequency, with no mid-diastolic potentials in electrograms of the ablation channel. The middle arrow indicates the exact point where successful ablation is obtained. LVindicates left ventricle.
Long-Term Follow-up
In the LocaLisa group, mean follow up lasted 10±8 months (range, 3-24 months). Of 7 patients whose RA was initially successful, 3 (42%) presented recurrence of clinical VT. In 2 of these patients, recurrence was early (within 48 hours) and a second procedure was successfully carried out. The remaining patient had a recurrence after 2 months of follow up, so antiarrhythmic medication was optimized. On the other hand, there were no recurrences in patients in whom RA was unsuccessful. In this group, 3 patients received an implantable cardioverter defibrillator (ICD) before the study and all remained free from events. Two patients required implantation of an ICD after the procedure due to induction of rapid VT, and neither patient presented recurrences.
In the control group, mean follow up was 19±8 months (range, 4-31 months). Four patients had received an ICD previously, whereas 7 had a device implanted after the procedure. After successful RA, 5 out of 14 patients (35%) had recurrence of VT in the 6 months after the procedure. In 3 patients, the VT was successfully ablated in a second procedure, whereas the other 2 patients presented VT episodes that were satisfactorily treated with the ICD by antitachycardia pacing. No deaths occurred in either group during follow-up.
DISCUSSION
Main Findings
There were slightly more successful procedures in the LocaLisa group, although this difference was not statistically significant. Nevertheless, the tendency towards more frequent VT with poor tolerance and the greater use of mapping during sinus rhythm in the LocaLisa group suggests that the procedure was more complex in these patients. In any case, given the small number of patients, it is impossible to discard a substantial effect of the system on the outcome of the procedure.
On the other hand, it is surprising that fluoroscopy time was not reduced in the LocaLisa group compared to control, as reported in a study of a series of patients who underwent RA of other arrhythmic substrates with this system. As mentioned earlier, however, our use of the system did not aim to reduce fluoroscopy time. Instead, we aimed to use the system to better define and locate the arrhythmic circuit. Also, the special characteristics of the arrhythmic VT substrate, in which a three-dimensional portion of the circuit is mapped, are different to other substrates such as atrial flutter, in which two-dimensional mapping of the circuit is performed. This might also have influenced the results because, like other systems, neither the path nor the curves of the probe catheter are displayed, and this is essential information for positioning at distant points within a heart chamber.
The new version of software includes the possibility of simultaneously displaying 2 different planes, which could help to reduce the radioscopy time and procedure duration.
Usefulness of the LocaLisa System
The present study reports our initial clinical experience with the three-dimensional nonfluoroscopic navigation system LocaLisa for RA of VT in patients with structural heart disease. The LocaLisa system has already proved useful in RA of certain atrial arrhythmogenic substrates. Kirchhof et al9 reported a 35% decrease in fluoroscopy time in ablation of supraventricular tachycardias after a short training period, and Schneider et al6 reported a similar reduction for RA of common atrial flutter. It has also been found to be useful in both isolation of pulmonary veins and RA of incisional atrial tachycardias.7,8 The system allows three-dimensional navigation of the catheter without displaying endocardial electrical activity and this may be sufficient for RA of well defined substrates that permit a purely anatomical approach. In complex substrates, the system may be more useful for RA of the substrate during sinus rhythm than for defining the circuit during tachycardia because this requires detailed mapping of endocardial activation combined with pacing to locate the critical area responsible for sustaining the reentry.18-22 However, our study was not designed to investigate this hypothesis and so cannot provide an answer. One advantage of the LocaLisa system over other systems is that it does not require an additional catheter or a specific navigation catheter. The cost is therefore lowered and, because implementation is simple, the duration of the procedure is not unduly affected. The system also facilitates repositioning of the RA catheter at specific sites. An additional advantage is that, unlike other systems, the catheter can be represented by averaging its position over the cardiac and respiratory cycles or faithfully reproducing its movements during the course of both cycles. Finally, in such patients, particularly those with scarring near the base of the heart, it is very useful to be able to guide the catheter from a cranial projection. A two-dimensional projection, which is impossible with fluoroscopy, can be used in patients with arrhythmic substrates near the base of the heart (Figure 2).
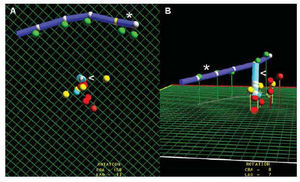
Fig. 2. Ablation of ventricular tachycardia after inferior myocardial infarction. A: oblique anterior left cranial projection showing different points of interest in the mapping. Yellow points represent mapping positions related to the entry point, exit point and slow conduction zone of the circuit. Red points represent sites of radiofrequency application. B: image of these points in anteroposterior projection. *indicates catheter implanted in coronary sinus; <, ablation catheter.
Limitations of the LocaLisa System
Although mapping and RA of VT were initially only performed under fluoroscopic navigation, several sophisticated three-dimensional visualization techniques have recently been developed.2-5 Important studies of RA of VT with different navigation systems such as the CARTO and EnSite system have recently been reported.23-27 These methods, unlike the LocaLisa system, provide both electrical and anatomical information. Combination of analysis of electrogram voltages and activation sequences helps to define the characteristics of the protected isthmus (entry point, slow conduction zone and exit point). Some systems even allow ablation of the VT circuit where conventional mapping is difficult or impossible. This is perhaps the main disadvantage of the LocaLisa system.23-28
This limitation of the system does not allow ready visualization of the spatial arrangement of the points and the probe catheter. Thus, we found the implantation of a multipolar catheter in the coronary sinus as an anatomical reference particularly useful because it facilitated mapping. In our study, only the last patients of the series had such a catheter implanted. Moreover, an anatomical display accurately defining the extent of scarring or activation of the overall activation of the left ventricle was not necessary as visualization of scarring in the slow conduction zone sufficed. This display can be readily constructed with the LocaLisa system. As in other three-dimensional navigation systems, a further limitation is that the reference electrode may move causing displacement in the same direction of the display of the points of the system with respect to its true anatomical location. This potential limitation, which we have seen with the use of the system for other arrhythmic substrates, did not occur in any of our patients with VT.
LIMITATIONS
This study describes initial experience of ablation of VT substrates with this technology and, as such, it is merely descriptive. The findings are limited by the low number of patients, low statistical power, and a learning curve for use of the LocaLisa system with this specific substrate. A potential advantage of the LocaLisa system is the shorter fluoroscopy time. A reduction in fluoroscopy time was, however, not a specific objective of our study, limiting the evaluation of one of the potentially most important advantages of the system. Also, although the results of the study group are controlled, enrollment was initially open and then changed to correlative and nonrandomized in the last patients.
CONCLUSIONS
In this essentially descriptive study, the LocaLisa system has proved able to define the reentry circuit and allow accurate repositioning of the ablation catheter at points of interest identified by mapping. This may be an advantage with respect to conventional techniques.
Correspondence: Dr. J.L. Merino.
Unidad de Arritmias y Electrofisiología (1.a PRE).Hospital La Paz.
P.o de la Castellana, 261. 28046 Madrid. España.
E-mail: jlmerino@secardiologia.es