The PARIS score allows combined stratification of ischemic and hemorrhagic risk in patients with ischemic heart disease treated with coronary stenting and dual antiplatelet therapy (DAPT). Its usefulness in patients with acute coronary syndrome (ACS) treated with ticagrelor or prasugrel is unknown. We investigated this issue in an international registry.
MethodsRetrospective multicenter study with voluntary participation of 11 centers in 6 European countries. We studied 4310 patients with ACS discharged with DAPT with ticagrelor or prasugrel. Ischemic events were defined as stent thrombosis or spontaneous myocardial infarction, and hemorrhagic events as BARC (Bleeding Academic Research Consortium) type 3 or 5 bleeding. Discrimination and calibration were calculated for both PARIS scores (PARISischemic and PARIShemorrhagic). The ischemic-hemorrhagic net benefit was obtained by the difference between the predicted probabilities of ischemic and bleeding events.
ResultsDuring a period of 17.2 ± 8.3 months, there were 80 ischemic events (1.9% per year) and 66 bleeding events (1.6% per year). PARISischemic and PARIShemorrhagic scores were associated with a risk of ischemic events (sHR, 1.27; 95%CI, 1.16-1.39) and bleeding events (sHR, 1.14; 95%CI, 1.01-1.30), respectively. The discrimination for ischemic events was modest (C index = 0.64) and was suboptimal for hemorrhagic events (C index = 0.56), whereas calibration was acceptable for both. The ischemic-hemorrhagic net benefit was negative (more hemorrhagic events) in patients at high hemorrhagic risk, and was positive (more ischemic events) in patients at high ischemic risk.
ConclusionsIn patients with ACS treated with DAPT with ticagrelor or prasugrel, the PARIS model helps to properly evaluate the ischemic-hemorrhagic risk.
Keywords
Dual antiplatelet therapy (DAPT) is one of the cornerstones used in the treatment of acute coronary syndrome (ACS).1 Combining DAPT with aspirin and a P2Y12 inhibitor has helped reduce the recurrence of ischemic events after ACS.2–4 However, use of this combination increases the bleeding risk, thus also worsening the prognosis.5 Achieving balanced antithrombotic therapy that maximizes the benefits of use (lowering ischemic risk without raising bleeding risk) is one of the objectives of current clinical cardiology in patients with ACS.6,7
Currently, several scores are used to stratify the risk of ischemic events.8 In patients at high ischemic risk, the current treatment guidelines for ACS recommend using more potent antiplatelets (ticagrelor or prasugrel) for longer times (< 12 vs ≥ 12 months).9–11 However, this recommendation should also be based on individual bleeding risk.7 Until recently, there have been no quantitative tools to help clinicians with postdischarge bleeding risk stratification in ACS. There are now several tools, such as the PARIS12 and PRECISE-DAPT13 scales, among others.14 However, because ischemic and bleeding risks often share predictors, it can be difficult to apply earlier tools in clinical decision-making.
The investigators of the PARIS registry (Patterns of Nonadherence to Antiplatelet Regimens in Stented Patients)12 recently attempted to resolve these gaps. The new PARIS score combines both risks in a single tool and is intended to aid clinicians in predicting postdischarge ischemic and bleeding risk in ACS.12
The aim of this study was to analyze the usefulness of the PARIS ischemic-hemorrhagic scale in a population of ACS patients percutaneously revascularized by coronary stenting (bare-metal and/or drug-eluting) who received DAPT with aspirin plus prasugrel or aspirin plus ticagrelor at hospital discharge.
METHODSStudy PopulationThe RENAMI (REgistry of New Antiplatelet therapy in patients with acute Myocardial Infarction) registry is a retrospective, observational, multicenter, international registry with voluntary participation of 11 sites in 6 European countries (Spain, Italy, Switzerland, Greece, Serbia, and the United Kingdom). This registry is an unfunded, investigator-dependent registry that arose from the need to learn more about the clinical benefit (ischemic-hemorrhagic risk) of DAPT with ticagrelor vs prasugrel in ACS. At the European Society of Cardiology congress held in 2016, the registry was suggested at a meeting of young investigators, and the following inclusion criteria were established: a) consecutive patients discharged with a diagnosis of ACS during any period between January 2012 and January 2016; b) with coronary stenosis ≥ 50% in the left coronary trunk or ≥ 70% in the rest of the coronary tree; c) treated by coronary stenting during the index hospitalization, and d) treated at hospital discharge with DAPT: aspirin (100 mg every 24 hours) plus prasugrel (10 mg every 24 hours) or aspirin plus ticagrelor (90 mg every 12 hours).
A database was designed for retrospective collection of information on clinical, laboratory, angiographic, and follow-up variables (death, ischemic and hemorrhagic events) (Table 1 of the supplementary material). The above databases from each of the 11 participating sites (Table 2 of the supplementary material) were sent in encrypted format to the coordinating site (Hospital Universitario Álvaro Cunqueiro in Vigo, Pontevedra, Spain) and then merged into a single registry. Two investigators at the coordinating site (E. Abu-Assi and S. Raposeiras-Roubín) were responsible for constructing the combined registry. The study was conducted in accordance with the Declaration of Helsinki, and approval was obtained from the local ethics committees.
The study classified ACS as ST-segment elevation acute myocardial infarction (AMI), non–ST-segment elevation AMI, and unstable angina.10,11
In this study, patients were excluded from the 4424 patients included in the RENAMI registry (based on the original design of the PARIS score, which focuses on events after discharge) in the following cases: a) BARC (Bleeding Academic Research Consortium) type 3 or 5 major bleeding during hospitalization (n = 52 [1.2%])15 and b) spontaneous in-hospital AMI16 or stent thrombosis (probable or confirmed)17 (n = 62 [1.4%]).
The final cohort for this study consisted of 4310 patients. All diagnostic and therapeutic procedures were performed according to local practices.
Objective, Definition, and Follow-upThe aim of the study was to evaluate the clinical usefulness of the PARIS score in assessing the ischemic-hemorrhagic net benefit in patients receiving DAPT with ticagrelor or prasugrel, as well as the predictive capacity of each score (PARISischemic and PARIShemorrhagic) to assess the risk of reinfarction/stent thrombosis and major bleeding.
Events were defined in this study according to the definitions adopted in the PARIS study.12 Major bleeding was considered to be BARC type 3 or 5 bleeding, whereas ischemic events were considered to be spontaneous AMI (defined as elevated myocardial injury markers above the upper limit of normal in combination with anginal symptoms or electrocardiographic abnormalities consistent with myocardial ischemia16) and confirmed or probable stent thrombosis according to the Academic Research Consortium.17
Information on events during follow-up was obtained from hospital and administrative medical records. The study only considered the first ischemic and bleeding event. The follow-up time was considered to have ended when the patient experienced an ischemic (n = 80 [1.8%]) or bleeding (n = 66 [1.5%]) event, death (n = 97 [2.3%]), DAPT suspension/withdrawal (n = 2609 [60.5%]), or end of follow-up in the local clinical registry.
Calculation of the Risk Scales of the PARIS Scale and CategorizationThe PARISischemic and PARIShemorrhagic scores were calculated according to the definitions used in the developmental cohort12 (Table 3 of the supplementary material).
Statistical AnalysisContinuous variables are shown as mean ± standard deviation. Discrete variables are expressed as percentages. Continuous variables were compared using the Student t test, and discrete variables were compared using the Pearson chi-square test. To analyze the incidence of events (ischemic and bleeding), cumulative incidence curves were plotted.
The individual components of the PARIS score were determined and found to be independent predictors in our series. To do this, these components were introduced in a Fine-Gray competing-risks regression model,18 with death as the competing event, to examine their association with the events studied (both ischemic and bleeding). The extent of this association is expressed by the subhazard ratio (sHR) and the respective 95% confidence interval (95%CI). The predictive capacity of the 2 PARIS scores (PARISischemic and PARIShemorrhagic) was also assessed by a Fine-Gray competing-risk regression model, using a similar approach to that described above. For comparisons between groups, the low-risk category was taken as a reference. To take into account the grouping of patients within each hospital, the Stata cluster option was used to perform a nonhierarchical cluster analysis.
The predictive capacity of the final model was calculated by the C statistic, using the c-index function of the pec extension for R. The calibration was assessed by comparing the observed and predicted probability in the 3 risk groups originally established in the referral cohort of the PARIS score,12 and by indicating the P values obtained by applying the Hosmer-Lemeshow test.
The ischemic-hemorrhagic net benefit was calculated as the absolute difference between the predicted probabilities for ischemic events and bleeding events, analogous to the approach taken in the referral cohort for the PARIS score.13 The expected risk of ischemic and bleeding events for both scores of the PARIS scale were modeled by polynomial fractions. Positive differences indicate excess ischemic risk, whereas negative differences indicate excess bleeding risk.
The predictive capacity of both scores for mortality was analyzed by a Cox regression model (Figure 1 of the supplementary material, Figure 2 of the supplementary material, Figure 3 of the supplementary material, and Figure 4 of the supplementary material). The linearity requirement for the 2 PARIS scores was confirmed by the Stata nlcheck command.
In the RENAMI registry, complete data were available for all variables except baseline hemoglobin and serum creatinine (3.3% of lost values, n = 141) and height and weight (16.6% of lost values, n = 717). Lost values were replaced with the medians of each variable according to sex. To assess the impact of lost data on the predictive capacity of the score, the sensitivity was analyzed after exclusion of patients with lost values (Table 4 of the supplementary material and Table 5 of the supplementary material).
Statistical analyses were performed using Stata/MP 13.1 and R 3.3.1. Results were considered statistically significant if P < .05.
RESULTSStudy Population and EventsThe mean age of the 4310 patients studied was 60.9 ± 11.5, and 20.8% were women. In all, 58.0% had ST-segment elevation AMI; 32.9% had AMI without ST-segment elevation, and 9.0% had unstable angina; 11.1% were in Killip class ≥ II.
A total of 61.4% (n = 2647) were treated with DAPT with ticagrelor. Six-month GRACE score data were available for 1528 (35.4%) patients, with a value of 120.7 ± 32.1 points. The mean GRACE score was 118.3 ± 32.1 points in patients with non–ST-segment elevation ACS and 122.7 ± 31.9 in patients with ST-segment elevation AMI.
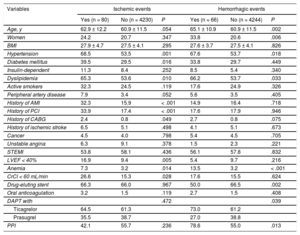
During 17.2 ± 8.3 months, 97 (2.3%) patients died; 80 experienced ischemic events (cumulative incidence of 1-year events, 1.9%; 95%CI, 1.5%-2.3%), of which 41 were stent thrombosis; 66 patients experienced BARC type 3 or 5 bleeding (cumulative incidence of 1-year events, 1.6%; 95%CI, 1.2-2.0%). Table 1 compares the baseline characteristics of patients with and without ischemic and bleeding events.
Baseline Characteristics of the Study Population According to Patient Group With and Without Events (Both Hemorrhagic and Ischemic)
| Variables | Ischemic events | Hemorrhagic events | ||||
|---|---|---|---|---|---|---|
| Yes (n = 80) | No (n = 4230) | P | Yes (n = 66) | No (n = 4244) | P | |
| Age, y | 62.9 ± 12.2 | 60.9 ± 11.5 | .054 | 65.1 ± 10.9 | 60.9 ± 11.5 | .002 |
| Women | 24.2 | 20.7 | .347 | 33.8 | 20.6 | .006 |
| BMI | 27.9 ± 4.7 | 27.5 ± 4.1 | .295 | 27.6 ± 3.7 | 27.5 ± 4.1 | .826 |
| Hypertension | 68.5 | 53.5 | .001 | 67.6 | 53.7 | .018 |
| Diabetes mellitus | 39.5 | 29.5 | .016 | 33.8 | 29.7 | .449 |
| Insulin-dependent | 11.3 | 8.4 | .252 | 8.5 | 5.4 | .340 |
| Dyslipidemia | 65.3 | 53.6 | .010 | 66.2 | 53.7 | .033 |
| Active smokers | 32.3 | 24.5 | .119 | 17.6 | 24.9 | .326 |
| Peripheral artery disease | 7.9 | 3.4 | .052 | 5.6 | 3.5 | .405 |
| History of AMI | 32.3 | 15.9 | < .001 | 14.9 | 16.4 | .718 |
| History of PCI | 33.9 | 17.4 | < .001 | 17.6 | 17.9 | .946 |
| History of CABG | 2.4 | 0.8 | .049 | 2.7 | 0.8 | .075 |
| History of ischemic stroke | 6.5 | 5.1 | .498 | 4.1 | 5.1 | .673 |
| Cancer | 4.5 | 4.0 | .798 | 5.4 | 4.5 | .705 |
| Unstable angina | 6.3 | 9.1 | .378 | 1.5 | 2.3 | .221 |
| STEMI | 53.8 | 58.1 | .436 | 56.1 | 57.8 | .832 |
| LVEF < 40% | 16.9 | 9.4 | .005 | 5.4 | 9.7 | .216 |
| Anemia | 7.3 | 3.2 | .014 | 13.5 | 3.2 | < .001 |
| CrCl < 60 mL/min | 26.6 | 15.3 | .028 | 17.6 | 15.5 | .624 |
| Drug-eluting stent | 66.3 | 66.0 | .967 | 50.0 | 66.5 | .002 |
| Oral anticoagulation | 3.2 | 1.5 | .119 | 2.7 | 1.5 | .408 |
| DAPT with | .472 | .039 | ||||
| Ticagrelor | 64.5 | 61.3 | 73.0 | 61.2 | ||
| Prasugrel | 35.5 | 38.7 | 27.0 | 38.8 | ||
| PPI | 42.1 | 55.7 | .236 | 78.6 | 55.0 | .013 |
AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass graft; CrCl, creatinine clearance; DAPT, dual antiplatelet therapy; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; STEMI, ST-segment elevation myocardial infarction.
Unless otherwise indicated, the data are expressed as percentages.
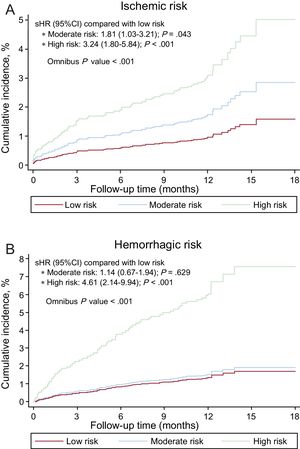
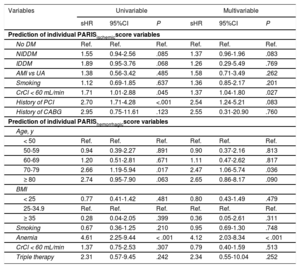
The PARISischemic score was significantly associated with ischemic risk, both continuously (sHR = 1.27; 95%CI, 1.16-1.39; P < .001) and categorically (Figure 1A). The PARIShemorrhagic score was also significantly associated with hemorrhagic risk, particularly continuously (sHR = 1.14; 95%CI, 1.01-1.30; P = .038). As a categorical variable, the moderate risk group showed no differences in the incidence of bleeding compared with the low-risk group (Figure 1B).
Table 2 shows the association of variables that comprise the PARIS risk scores with ischemic and bleeding events.
Univariable and Multivariable Analysis of PARIS Score Variables
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| sHR | 95%CI | P | sHR | 95%CI | P | |
| Prediction of individual PARISischemicscore variables | ||||||
| No DM | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| NIDDM | 1.55 | 0.94-2.56 | .085 | 1.37 | 0.96-1.96 | .083 |
| IDDM | 1.89 | 0.95-3.76 | .068 | 1.26 | 0.29-5.49 | .769 |
| AMI vs UA | 1.38 | 0.56-3.42 | .485 | 1.58 | 0.71-3.49 | .262 |
| Smoking | 1.12 | 0.69-1.85 | .637 | 1.36 | 0.85-2.17 | .201 |
| CrCl < 60 mL/min | 1.71 | 1.01-2.88 | .045 | 1.37 | 1.04-1.80 | .027 |
| History of PCI | 2.70 | 1.71-4.28 | <.001 | 2.54 | 1.24-5.21 | .083 |
| History of CABG | 2.95 | 0.75-11.61 | .123 | 2.55 | 0.31-20.90 | .760 |
| Prediction of individual PARIShemorrhagicscore variables | ||||||
| Age, y | ||||||
| < 50 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 50-59 | 0.94 | 0.39-2.27 | .891 | 0.90 | 0.37-2.16 | .813 |
| 60-69 | 1.20 | 0.51-2.81 | .671 | 1.11 | 0.47-2.62 | .817 |
| 70-79 | 2.66 | 1.19-5.94 | .017 | 2.47 | 1.06-5.74 | .036 |
| ≥ 80 | 2.74 | 0.95-7.90 | .063 | 2.65 | 0.86-8.17 | .090 |
| BMI | ||||||
| < 25 | 0.77 | 0.41-1.42 | .481 | 0.80 | 0.43-1.49 | .479 |
| 25-34.9 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ≥ 35 | 0.28 | 0.04-2.05 | .399 | 0.36 | 0.05-2.61 | .311 |
| Smoking | 0.67 | 0.36-1.25 | .210 | 0.95 | 0.69-1.30 | .748 |
| Anemia | 4.61 | 2.25-9.44 | < .001 | 4.12 | 2.03-8.34 | < .001 |
| CrCl < 60 mL/min | 1.37 | 0.75-2.53 | .307 | 0.79 | 0.40-1.59 | .513 |
| Triple therapy | 2.31 | 0.57-9.45 | .242 | 2.34 | 0.55-10.04 | .252 |
AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass graft; 95%CI, 95% confidence interval; CrCl, creatinine clearance; DM, diabetes mellitus; IDDM, insulin-dependent diabetes mellitus; NIDDM, noninsulin-dependent diabetes mellitus; PCI, percutaneous coronary intervention; sHR, subhazard ratio; UA, unstable angina.
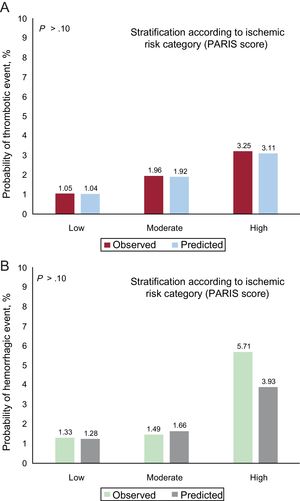
The discriminatory capacity of the PARIS score for ischemic events was modest (C statistic = 0.64; 95%CI, 0.62-0.66), with good calibration (Figure 2A), but was suboptimal for bleeding events (C statistic = 0.56; 95%CI, 0.53-0.59), with acceptable calibration, particularly for low- and moderate-risk groups (Figure 2B).
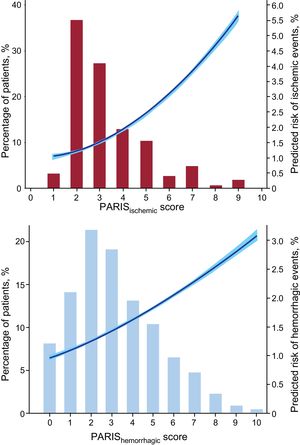
Figure 3 shows the distribution of both PARIS scores, with the predicted probability of the respective events. Most patients were classified as low-to-moderate ischemic risk (≤ 4 points) and low hemorrhagic risk (≤ 3 points). Only a few patients were classified as high hemorrhagic risk (n = 280 [6.5%]).
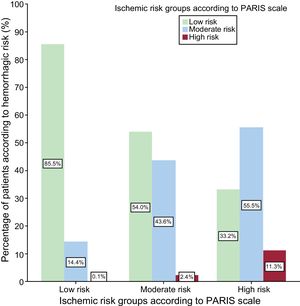
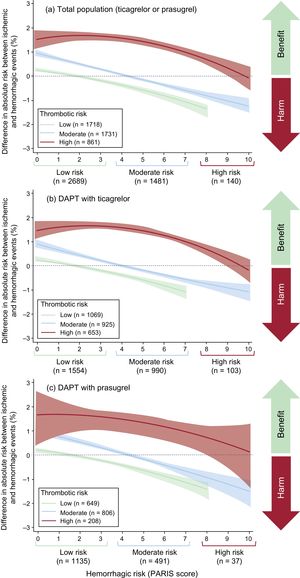
Ischemic-hemorrhagic BalanceMost (85.5%) patients at low ischemic risk also had low hemorrhagic risk according to the PARIS scale. However, only 11.3% of patients at high ischemic risk were at high hemorrhagic risk (Figure 4).
Figure 5 contains a plot of the ischemic-hemorrhagic net benefit according to the patient's ischemic and hemorrhagic risk group. The ischemic-hemorrhagic net benefit was negative (higher risk of bleeding vs ischemic events) for most patients at low ischemic risk (higher as hemorrhagic risk increases), as well as for patients at moderate ischemic risk and moderate-to-high hemorrhagic risk.
In patients at high ischemic risk, the ischemic-hemorrhagic net benefit tended to be positive, particularly for those at low-to-moderate hemorrhagic risk. These results are similar to those obtained for ticagrelor and for prasugrel (Figure 5).
DISCUSSIONThis study demonstrates the usefulness of the PARIS scale to optimize ischemic-hemorrhagic net benefit in a population of ACS patients treated by percutaneous coronary intervention who received DAPT with ticagrelor or prasugrel. The information in this international registry, which has more than 4000 real-life patients, validates the 2 risk scores (ischemic and hemorrhagic) of the PARIS scale. The discriminatory capacity of ischemic events was modest, whereas that of hemorrhagic episodes was poor but well calibrated.
Although ischemic and hemorrhagic risk are closely correlated,19 the results of this study indicate that, based on the PARIS scale, patients with discordant risks can be identified. In fact, combining both scores (PARISischemic and PARIShemorrhagic) enables prediction of the ischemic-hemorrhagic net benefit. After stratifying patients at low, moderate, and high risk, we observed that patients at low ischemic risk and patients at high hemorrhagic risk usually have a negative ischemic-hemorrhagic net benefit (more bleeding than ischemic events) with DAPT with prasugrel and ticagrelor, whereas patients at high ischemic risk and patients at low hemorrhagic risk have a positive ischemic-hemorrhagic net benefit (more ischemic than bleeding events). In patients at moderate ischemic risk, the ischemic-hemorrhagic balance depends on hemorrhagic risk: this balance tends to be negative in patients at moderate-to-high hemorrhagic risk and positive in patients at low hemorrhagic risk.
These results should be interpreted in the clinical context of the study: patients treated by percutaneous coronary intervention who were prescribed DAPT consisting of ticagrelor or prasugrel at discharge, who had no ischemic or bleeding events during hospitalization, and who had a moderate 6-month risk of death as estimated by the GRACE scale.
The PARIS predictive scale12 has been well received for evaluating the risk-benefit of DAPT. For instance, the review included in the U.S. guidelines on DAPT duration20 recommends evaluating the bleeding risk and adding it to the ischemic risk in a similar manner as the PARIS scale. Therefore, patients predicted to have a negative ischemic-hemorrhagic net benefit due to the predominance of bleeding events are advised to take shorter courses of DAPT, preferably with clopidogrel instead of ticagrelor or prasugrel.11
In the original PARIS scale12, 2-year bleeding rates < 2% were observed in patients at low hemorrhagic risk, 2% to 4% in patients at moderate risk, and > 4% in patients at high risk. In our study, in a patient population prescribed DAPT with ticagrelor or prasugrel, the cumulative incidence of 1-year bleeding events was very similar between patients at moderate or low risk of bleeding (both below 2%) and patients at high risk of bleeding (> 4%), although the discriminatory capacity of the PARIShemorrhagic score was rather poor. A possible explanation for this modest result in the prediction of only bleeding events is that the population of our study (RENAMI) is basically a selected population. These patients were prescribed DAPT with ticagrelor or prasugrel instead of clopidogrel at the attending physician's discretion. Moreover, prescription was usually influenced by a subjective estimate of low-to-moderate hemorrhagic risk. In fact, prasugrel is not recommended for patients older than 75 years, a history of stroke, or weight < 60 kg because DAPT with prasugrel in these patients has been associated with a higher risk of bleeding and an unfavorable ischemic-hemorrhagic net benefit compared with DAPT with clopidogrel.2
Once the 2 scores of the PARIS scale were combined and stratified by risk group, the estimate of the ischemic-hemorrhagic net benefit in this population was good. The results have shown that it is possible to consider using the PARIS scale (designed in a population that mainly received DAPT with clopidogrel, with barely 7% of patients receiving prasugrel [n < 400] and none receiving ticagrelor) to determine the ischemic-hemorrhagic net benefit in patients treated with DAPT with ticagrelor and prasugrel. Because the ischemic-hemorrhagic profile of clopidogrel differs considerably from that of ticagrelor or prasugrel,2,3 the ischemic-hemorrhagic net benefit is useful to identify the best antithrombotic strategy at discharge in terms of DAPT duration and type.21,22 Thus, for patients at high hemorrhagic risk according to the PARIS score, particularly if the ischemic risk is low to moderate, not only is it necessary to recommend shorter courses of DAPT,23–26 but also to use DAPT with clopidogrel instead of ticagrelor or prasugrel.2,3,27 Although the PARIS scale was derived from a patient population with ischemic heart disease (both stable angina and ACS, which accounted for 37.8% of the PARIS population) treated with drug-eluting stents,12 the results focus only on ACS patients, all of them treated with drug-eluting or bare-metal stents.
It should be taken into account that in the past 2 years, various risk scores have been proposed to help physicians make decisions regarding the type and duration of DAPT. Recently, after the PARIS score, the PRECISE-DAPT score13 was created to try to stratify patient risk and the benefit of shortening or lengthening DAPT duration according to hemorrhagic risk. The advantage of the PARIS score,12 recommended by the U.S. guidelines, over the PRECISE-DAPT score,13 recommended by the European guidelines, is that both risks (ischemic and hemorrhagic) are combined, making it possible to calculate the expected ischemic-hemorrhagic net benefit.
Clinicians can also use the 1-year DAPT score27 to help select patients who will benefit from using DAPT beyond the first year. Compared with the DAPT score, the PARIS score12 has the advantage that it allows the ischemic-hemorrhagic balance to be estimated at discharge rather than after 1 year, thus indicating when TAPD should be prolonged or when the patient should have a shorter course.23,24
LimitationsThe main limitation of the present study is its retrospective nature, with the inherent limitations of this type of registry. As mentioned in the discussion, there is considerable therapeutic selection bias in the population because all patients had been prescribed DAPT with prasugrel or ticagrelor instead of clopidogrel according to the guidelines and preferences at each site and at the attending physician's discretion, primarily limited by the subjective estimate of a low or moderate bleeding risk. Additionally, the study excluded patients with ischemic and hemorrhagic events during hospitalization. Moreover, the results of the present study should be interpreted in the context of patients at moderate risk according to the GRACE score used to predict 6-month mortality. Another limitation of this study is that there were no data on the vascular access used for inpatient coronary angiography. Likewise, the findings can be generalized only to ACS patients treated with percutaneous coronary intervention during hospitalization and at discharge with DAPT with prasugrel or ticagrelor plus aspirin. Despite these limitations, the study supports the hypothesis that the PARIS ischemic-hemorrhagic scale could be useful when deciding the type and duration of DAPT after ACS. A prospective study should be conducted to determine whether DAPT should be personalized to each patient according to the PARIS scale to prevent ischemic and hemorrhagic events.
CONCLUSIONSIn ACS patients treated by percutaneous coronary intervention during hospitalization and DAPT with ticagrelor or prasugrel at discharge, the PARIS scale could be useful to estimate ischemic-hemorrhagic net benefit, thus helping to determine the best antithrombotic strategy for each patient in terms of DAPT type and duration.
CONFLICTS OF INTERESTE. Abu-Assi is associate editor of Revista Española de Cardiología.
- –
The PARIS score has been developed in a population of patients with ischemic heart disease (< 50% ACS) treated by percutaneous revascularization with a drug-eluting stent and dual antiplatelet therapy (> 90% with clopidogrel). In addition to demonstrating adequate predictive capacity for both ischemic and bleeding events, its advantage largely lies in its capacity to combine both risks in a single patient, making it easier to assess ischemic-hemorrhagic net benefit more precisely and allowing the best antithrombotic strategy to be identified in terms of DAPT type and duration.
- –
This study is the first to analyze the capacity of the PARIS score to predict ischemic and bleeding events in a population of ACS patients treated by percutaneous revascularization (drug-eluting and bare-metal stents) and dual antiplatelet therapy with ticagrelor or prasugrel. The usefulness of the PARIS score to combine both risks (ischemic and hemorrhagic) was demonstrated in this population, identifying patient groups with a more favorable ischemic-hemorrhagic net benefit and potentially benefiting from dual antiplatelet therapy with ticagrelor and prasugrel.