To the Editor,
The persistence of patent foramen ovale (PFO) in adults has been correlated with an increased incidence of cryptogenic stroke or strokes of an undetermined etiology. Percutaneous PFO closure in high-risk patients or patients with recurrent strokes in spite of medical treatment is a feasible, safe, and efficient technique.1
Here, we present the case of a 52-year-old woman, with a history of migraines with auras, who came to the hospital due to a sudden left hemiparesia and right central facial paralysis with aphasia. The symptoms disappeared a few hours after admission and the patient recovered completely. After performing an echocardiogram and a 24-hour Holter test which showed sinus rhythm, a CT scan and nuclear magnetic resonance imaging showing no lesions, a complete coagulation study with normal results, a Doppler-Ultrasound of the supra-aortic trunks with no alterations, and a transthoracic echocardiogram showing the presence of PFO following bubble injection, the diagnosis of transient ischaemic attack (TIA) of an unknown etiology of the middle left cerebral artery was established.
This being the first episode of TIA, and since the patient had PFO with high-risk criteria, medical treatment with acetylsalicylic acid at 100 mg/ 24 h was initiated. At 5 months, the patient suffered another TIA, and we decided to close the PFO.
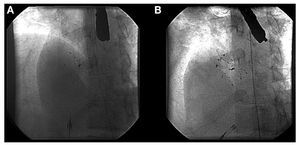
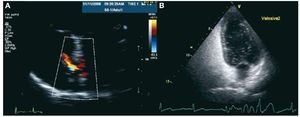
A 33 mm CardioSeal device (Figure 1) was implanted as usual, with no complications. However, the transthoracic echocardiogram taken before discharge (24 hrs after implantation) showed the presence of a moderate residual shunt (Figure 2). In the echocardiograms taken at one month and 6 months, we confirmed the persistence of the shunt, which had not reduced in size, and also observed the passage of bubbles using Valsalva maneuvers (Figure 2), and so we decided to perform a percutaneous closure of the residual shut. A transeptal puncture was performed in the area above the device, previously implanted under echocardiographic control, and an Amplatzer ASD 18 mm device was implanted (Figure 1). A complete closure was confirmed along with an absence of passage of bubbles one month and 6 months after the procedure. After 2 years of follow-up, the patient still has not had any recurrences, and the migraines have disappeared.
Figure 1. A: CardioSeal implant device (33 mm) for closing a patent foramen ovale. B: implant of a second 18 mm Amplatzer ASD device to close the residual shunt.
Figure 2. A: echocardiogram following the implantation of the first device showing the persistence of the residual shunt through a Doppler color image. B: echocardiogram following implantation of the first device showing the passage of bubbles through the patent foramen ovale during the Valsalva maneuvers.
The presence of at least a moderate residual shunt after a percutaneous PFO closure has been related to increased risk of recurrent stroke during follow-up.2,3 Complete PFO closure is achieved in 95% of cases, but the presence of moderate or severe shunts has been described in 2%-3%.3 An incomplete closure can be related to an under-sized device, an inadequate design of the device given the morphology of the PFO, or multiple septal fenestration. The treatment for this subgroup of patients is not clear: medical treatment can be continued, surgical closure can be considered, and recently, percutaneous closure of the residual shunt has been described.4,5
In the case we have presented, the second device implantation was performed with no complications using the transeptal puncture technique, which facilitates the placement of a second device in the area of the shunt. During the follow-up period of this high-risk patient, no recurrent cerebral strokes have been recorded.
Studies are required that include a greater number of patients in order to confirm the safety and efficacy of this technique.