Keywords
INTRODUCTION
Enlargement of the outflow tract is a basic concept in the surgical correction of heart disease with right ventricular obstruction, such as tetralogy of Fallot (TOF). Cases requiring annular enlargement involves the problem of pulmonary valve regurgitation (PVR). The combination of ventriculotomy and pulmonary regurgitation leads to the deterioration of right ventricular function and arrhythmias in the long term.1 Different methods of valve implantation (mechanical or biological prosthesis) or valved conduits (homografts and xenografts) solve the problem of PVR in the short- to medium term while solving the right outlet obstruction.2-9 However, given their biological character, tissue degeneration can be expected with subsequent stenosis and regurgitation over time.
A simple method to obtain pulmonary competence (in cases requiring a transannular patch [TAP] for surgical correction) is the implantation of an expanded polytetrafluoroethylene (PTFE) monocusp valve. This facilitates early recovery after surgery, and benefits can persist in the medium to long term. Other studies with pericardial monocusp valves have questioned their usefulness even in the short to medium term.3,7 Several technical modifications in the surgical implantation of PTFE valves in the pulmonary position have been reported.2,5-8,10,11 We present our initial experience using the technique proposed y Nunn et al in 2008.10
METHODS
In total, 21 consecutive patients who needed enlargement of the right outflow tract with a TAP were treated between September 2008 and February 2010. Of these, 19 had TOF and 2 had complete atrioventricular channel with TOF. Four patients had previously undergone palliative treatment with a PTFE systemic-pulmonary shunt via right thoracotomy. Their ages ranged from 7 months to 15 years (median, 12 months) and weights from 6.8 kg to 44 kg (median, 10 kg). After surgical correction, transesophageal echocardiography (TEE) was performed using a pediatric omniplane probe to determine infundibulum morphology and the degree of pulmonary regurgitation, classified as mild, moderate, or severe.12 In most of the patients, the pressure gradient was not determined due to difficulties in aligning the right ventricular outflow tract. The pressures were determined by direct puncture of the left and right ventricle. All the patients underwent echocardiography before discharge to determine the pulmonary gradient and the degree of pulmonary neovalvular regurgitation (Table).
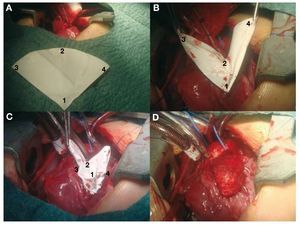
Following transatrial closure of the ventricular septal defect (VSD) and aortic declamping, the outflow tract was enlarged with a TAP (Figure 1). The fact of having 3 holding stitches allowed us to use the beating-heart technique. The pulmonary neovalve was a 90o-120o semicircle of 0.1-mm PTFA whose radius equaled the distance between the commissure of the native pulmonary valve and the lower vertex of the ventriculotomy incision.10 Its fan-like shape (Figure 1A), its most characteristic feature offers a very generous free edge compared to classic monocusp valves.2 The central point of the curved free edge (circular) is sutured to the posterior side of the native pulmonary artery in the commissural plane (Figure 1B). The vertex of the patch is tied to the vertex of the ventriculotomy incision and the two ends of the suture are used to join the straight sides of the patch to both edges of the ventriculotomy incision (Figure 1C). Finally, the TAP (glutaraldehyde-treated autologous pericardium) is fixed to the edges using an independent suture (Figure 1D), thereby covering the pulmonary neovalve (Figure 2).
Figure 1. A: fan-shaped expanded polytetrafluoroethylene (PTFE). B: suturing the vertex of the patch (A) and central point of the curved edge (B). C: suturing both straight edges of the patch to those of the ventriculotomy incision (C,D). D: transannular patch of autologous pericardium (covering the PTFE valve).
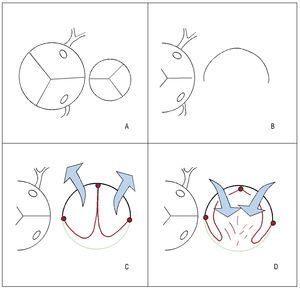
Figure 2. A: relative sizes of aortic and pulmonary valves in tetralogy of Fallot. B: pulmonary artery, open and flat. C: expanded polytetrafluoroethylene (PTFE, red) fixed at the central point and edges (in the shape of a 3) with a transannular patch (green) in systole (arrows, blue). D: PTFE valve (red) in diastole (arrows, blue), moving toward the perimeter of the pulmonary neoartery (native artery shown in black and patch of pericardium shown in green).
RESULTS
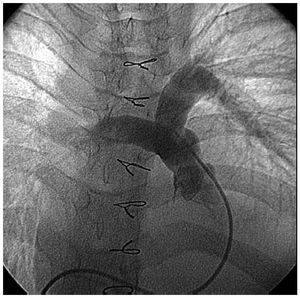
The intraprocedural TEE showed mild regurgitation in 19 patients and moderate in 2 (the first and second in our series). Pre-discharge echocardiography showed no change in the degree of pulmonary regurgitation. The residual gradient in the outflow tract fluctuated between 0 mmHg and 25 mmHg (Table), except in 2 patients in whom this reached 50 mmHg (the second in the series, with hypoplasia of the branches, and the fourth in the series, 15 years old, with a very restricted right ventricle with dynamic stenosis). One patient (the fourth in the series) presented residual interventricular communication which was designated as minimal early after surgery and underwent reintervention 6 months after correction. Previous catheterization showed minimal neopulmonary regurgitation that was classified as mild on TEE (Figure 3).
Figure 3. Pulmonary angiogram showing minimal regurgitation.
DISCUSSION
Currently, the early outcomes of surgical repair of TOF are considered excellent, regardless of timing and surgical technique.1,2,13 Inevitably, the TAP leads to pulmonary regurgitation. Subsequent physiopathological changes are well tolerated in the long term by most patients, although some may present right ventricular failure. The sharp changeover from pressure overload to volume overload associated with ventriculotomy can also lead to acute response early after surgery.1 The aim of using a pulmonary valve mechanism in the correction of TOF is twofold: better recovery early after surgery and, ideally, maintenance of valvular competence in the medium- to long term. One of the advantages of the in situ preparation of the surgical material is that it can be customized to the needs of the patient. In our case, both the shape and dimensions of the TAP and the PTFE pulmonary neovalve can be modified. The reason for fixing the central point of the curved edge to the commissural plane of the native pulmonary valve is to replicate an anchor point to optimize the closure mechanism.10 Thus, the minimum radius of the neovalve should be equal to the distance between this plane (immediately below the pulmonary bifurcation) and the vertex of the ventriculotomy incision. The calculation of the chord (curved free edge) and consequent angle of the circular sector remains a matter of debate. We can apply simple trigonometric formulas taking into account the final diameter we wish to obtain after placement of the TAP, the total length of the patch, and substraction of the amount of tissue taken up by the suture line, etc. In practice, a circular sector with an angle close to 120o obtained good outcomes in our series and others.10 In addition, the generous free edge could raise concerns regarding the possibility of obstruction; however, this did not occur due to the elasticity of the extremely thin material used (0.1 mm), such that platelet aggregation inhibitors were not needed.10,11
Valve mechanism: the distal fixation of the neovalve takes the shape of a 3 or double-arched vault, its operation resembling that of a bileaflet prosthesis with anteroposterior orientation (unlike the classic monocusp valve2 that has a right-left orientation). It is specifically the bileaflet configuration that halves the time it takes for the free edge (center-to-periphery) to move over the perimeter of the pulmonary artery (enlarged with the TAP) compared to the classic monocusp valves (in the former this is equivalent to the radius, in the latter it is equivalent to the diameter). This mechanism optimizes the opening and closing of the neovalve in systole and diastole, respectively10 (Figure 2). The high profile of the valve (from the vertex of the ventriculotomy incision almost up to the pulmonary bifurcation) causes a contained regurgitation phenomenon (not failure) similar to that of the sinuses of Valsalva. Echocardiographic measurement at the origin of the pulmonary branches will show a certain degree of regurgitation, but not so in the infundibulum. This can explain the discrepancy between the mild regurgitation (on echocardiography) and trivial regurgitation (by catheterization) in the same patient in our series who underwent reintervention (Figure 3). The issue of whether echocardiography overestimates the degree of regurgitation in these patients remains outside the aims of this study. The implantation technique is simple and takes little time. In standard practice, it is performed after declamping the aorta and checking the subaortic suture of the VSD after transatrial closure. Three holding stitches (vertex of the ventriculotomy incision and both edges of the pulmonary annulus) help stabilize the area although the heart is beating. If the left pulmonary artery needs enlarging, we fix the distal points of the TAP prior to declamping and then perform the procedure described. It is often oversized in case a TAP is required, thereby obtaining a pulmonary neoartery perimeter larger than necessary (equivalent to that of the aorta). Logically, this makes it possible to implant an equally oversized pulmonary neovalve. The larger its size at the time of implantation, the longer the duration of valvular competence. The possibility of permanent implantation in cases of post-procedural pulmonary regurgitation in young adults could be considered.10,11,13 The authors do not pretend to describe anything more than their initial experience with the technical variation described by Nunn et al10 in terms of complexity, reproducibility, and immediate results. Long-term follow-up of the series will confirm the durability of valvular competence (ideally, with magnetic resonance imaging studies). The study by Nunn et al10 demonstrated excellent late results in 93% and only trivial or mild pulmonary regurgitation compared to 50% in the Indianapolis group.2 The challenge remains interesting.
CONCLUSIONS
The implantation of a TAP with a modified monocusp valve to completely repair the outflow tract in TOF is simple and replicable. The initial results are promising; there was only mild regurgitation and a slight pressure gradient during the intraprocedural period and early after surgery. A medium or long-term follow-up study is required to confirm these findings and to compare them with results obtained using other techniques.
ABBREVIATIONS
PTFE: expanded polytetrafluoroethylene
PVR: pulmonary valve regurgitation
TAP: transannular patch
TEE: transesophageal echocardiography
TOF: tetralogy of Fallot
VSO: ventricular septal defect
Correspondence: Dr. J.M. Gil-Jaurena.
Jefe Sección Cirugía Cardiaca Infantil.
Hospital Materno-Infantil Carlos Haya.
Arroyo de los Ángeles, s/n. 29011. Málaga. Spain.
E-mail: giljaurena@gmail.com
Received March 23, 2010.
Accepted for publication June 16, 2010.