In 2017 an update was made to the consensus document on the clinical use of the polypill for secondary prevention in cardiovascular (CV) disease, which had been written in 2015 and published in Revista Española de Cardiología.1 This document was the first to establish recommendations on the use of this treatment option and had the scientific endorsement of the Spanish Society of Cardiology (SEC), the Spanish Society of Internal Medicine (SEMI), the Spanish Society of Family and Community Medicine (SemFYC), the Spanish Society of General and Family Practitioners (SEMG), and the Spanish Society of Primary Care Physicians (SEMERGEN).
There were multiple reasons for updating the document: a) the publication of new evidence on polypill use in everyday clinical practice, b) the introduction of a new dosage form with 40 mg atorvastatin in addition to the existing forms, and c) the recommendation in the document “La actualización del Manual Metodológico para la Elaboración de Guías de Práctica Clínica en el Sistema Nacional de Salud” (Update to the Methodological Manual for the Development of Clinical Practice Guidelines in the National Health System),2 issued in 2016, on the appropriateness of carrying out updates at least every 2 years, with the aim of maintaining the value of the guidelines, both for clinical staff and for health care regulators.
The update3 discussed here was carried out based on the methodological manual mentioned above, 2 which establishes the foundation that should guide the update of recommendations. Broadly, after a nonexhaustive systematic search for new evidence, a bibliographic synthesis was developed that allowed the recommendations update group to propose the new recommendations to be included and those requiring modification. The group that validated the recommendations (28 experts), using a modified Delphi method, proceeded to the validation of the new and modified recommendations; the percentage agreement was higher than that established in the working protocol (> 80%), and consequently a final participative session was not required. All the new and modified recommendations were categorized with the level of evidence and grade of recommendation, according to the modified version of the Scottish Intercollegiate Guidelines Network (SIGN) system.4
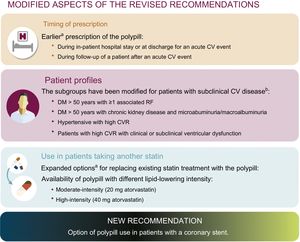
Of all the recommendations in the 2015 document, 32 were considered still valid, 5 were updated, none were removed, and 1 new recommendation was included. Figure 1 shows the topics updated, which can be summarized as: a) earlier prescription for patients with an acute CV event, b) the possibility of using the polypill for patients on high-intensity lipid-lowering therapy with a statin other than atorvastatin; c) redefinition of the patient profiles for high or very high risk and subclinical CV disease who could take the polypill, and d) use in patients with stents.
The update document does not contain any changes to the preferred indication criteria for the polypill in the context of secondary prevention of CV disease, although the new evidence on polypill use and the introduction of the new 40mg atorvastatin dosage form do broaden the therapeutic spectrum of patients who could benefit from its use. Furthermore, these developments justify the update to the consensus document on the clinical use of the polypill as CV risk prevention, which will allow clinical staff more uniformity in making decisions in line with the available evidence.
FUNDINGFerrer Internacional funded the logistics required for the document to be updated, but did not participate in the discussions or decision-making.
CONFLICTS OF INTERESTJ.R. González-Juanatey has received fees from Ferrer Internacional for giving lectures.
We are grateful for the work performed by the other members of the scientific committee (Benjamín Abarca, José María Lobos, José Luis Llisterri, and José María Mostaza), the recommendations update group (José Juan Alemán, Gonzalo Barón-Esquivias, Isabel Egocheaga, Enrique Galve, Francisco Xavier García-Moll, Rosa María Lidón, Jesús Millán, Vicente Pallarés-Carratalá, Pedro Luis Sánchez, and Carmen Suárez) and the recommendations validation group. We also thank Ferrer Internacional for their support and GOC Networking for their technical and methodological support.