Cardiac imaging is a cornerstone of diagnosis in heart conditions, and an essential tool for assessing prognosis and establishing treatment decisions. This year, echocardiography stands out as a guide in interventional procedures and in choosing the size of the prosthesis. It is also proving to be a valuable technique in low-flow, low-gradient aortic stenosis. Three-dimensional echocardiography is advancing our knowledge of cardiac anatomy and valvular measurements. The parameters indicating tissue deformation have predictive power in valve disease and in the follow-up of drug-induced cardiotoxicity. Single-photon emission computed tomography and positron emission tomography are proving useful in ischemic heart disease and in the diagnosis of cardiac inflammation and infections. The role of computed tomography has been strengthened in noninvasive coronary angiography, the emergency room management of chest pain, assessment of chronic occlusions, and morphologic study of coronary plaque. Cardiac magnetic resonance imaging remains the gold standard for tissue characterization in ischemic heart disease and cardiomyopathies, and is assuming a greater role in stress studies and in the assessment of myocardial viability.
Keywords
Few of the ongoing technological innovations in echocardiography have been incorporated into clinical practice. Applications are being developed for automatic motion measurement, evaluation of tissue deformation by 3-dimensional (3D) echocardiography,1 and classification of regional wall motion.2 Even more promising is the possibility of using transthoracic high-intensity focused ultrasound to perform ablation of cardiac structures without the need for surgery or catheters.3 Another related application that is already used in humans is integration of ultrasound capability in pacemakers to send signals to the endocardial leads wirelessly to regulate pacing.4 The incorporation of web-based networks for interpretation of studies conducted by paramedics is now a reality.5
Stress EchocardiographyThe use of stress echocardiography is not indicated in asymptomatic revascularized patients.6 Patients who achieve a normal submaximal stress echocardiography have a higher risk of events than those attaining maximal stress.7 Use of stress echocardiography to evaluate patients with dyspnea has shown that the incidence of induced ischemia is low and mainly appears in those with baseline contractility abnormalities.8
Contrast-Enhanced Myocardial PerfusionStress myocardial perfusion studies with contrast enhancement have proven useful for establishing patient prognosis and predicting events.9 This technique enhances detection of coronary disease and better identifies patients who will require revascularization.10
Cardiac Interventional ProceduresThe 3D transesophageal echocardiography is acquiring an increasingly more important role in interventional procedures. Routine inclusion of this technique in protocols for transcatheter aortic valve implantation improves outcomes11 and is an alternative to multislice computed tomography (CT); both techniques accurately estimate final sizing of the prosthesis.12 Use of a single view for assessing the aortic annulus can lead to errors in the classification of patients as unsuitable candidates for transcatheter aortic valve implantation.13 The presence of regurgitation around the prosthesis has a negative impact on the prognosis. Assessment of this feature is complex and requires various imaging techniques.14
Mitral repair with the use of MitraClip® leads to reverse remodeling of the left ventricle and annulus, with an improvement in myocardial function.15 Standardization of the imaging techniques is necessary in the assessment of periprosthetic dehiscences and in determining the choice of approach.16
In percutaneous left atrial appendage closure, insufficient oversizing when calculating the device size is associated with a greater probability of residual leakage.17 Transesophageal echocardiography study during follow-up enables detection of a considerable percentage of device-associated thrombi.18
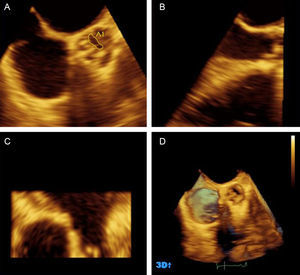
Valve DiseaseSevere low-flow, low-gradient aortic stenosis with preserved ejection fraction is a clinically relevant entity. Various studies have demonstrated the severity of this condition by hemodynamic study19 and 3D transesophageal echocardiography planimetry20 (Figure 1). The prognosis is poorer than that of severe, high-gradient stenosis,21,22 and aortic valve replacement is associated with increased survival.23 A key issue in evaluation is to avoid errors in classifying the severity of the condition. stress echocardiography can be helpful in the assessment.24 Some authors25 have proposed reducing the cut-off for severity to 0.8 cm2 for small (17-20 mm) left ventricular outflow tracts.
Three-dimensional echocardiography of the aortic valve. A-C, multiplanar reconstruction. A, transverse plane at the level of the cusps, where planimetry is performed. B, longitudinal plane used for selection of the transverse plane at the cusps. D, bicuspid aortic valve. 3D, 3-dimensional. Adapted with the permission of González-Cánovas et al.20.
In aortic stenosis associated with ventricular dysfunction, cases that are “pseudo-severe” have a better outcome with conservative management than those that are “truly severe”, and the results are comparable to those of ventricular dysfunction without valve disease.26 Therefore, surgical or percutaneous treatment of the valve disease is not supported in these patients.
Stress echocardiography can be useful in the management of asymptomatic mitral failure, since the right ventricular dysfunction induced during the procedure is associated with shorter valve surgery-free survival.27
Three-dimensional EchocardiographyIn patients with mitral valve prolapse, flattening of the annulus is reported to contribute to the pathogenesis of mitral regurgitation, by inducing progressive protrusion of the leaflets, which could lead to a greater risk of cord rupture.28
The development of automatic software has improved measurement of mitral regurgitation29 and assessment of the complete aorta-aortic valve complex, and this capability will be an aid in refining the related repair techniques.30
Recent publication of the largest study to date on the reference values for 3D echocardiography left ventricular volumes and function31 will surely contribute to wider application the technique in this line.
In addition, 3D echocardiography provides valuable information in complex anatomical situations, such as atrial septum anatomical variants.32
Aortic DiseaseConcerning the reference values and standardization of proximal thoracic aorta measurement, there is a need to correct not only for body surface area and age, but also for sex.33 Nonetheless, in Marfan syndrome, the use of the left ventricular outflow diameter vs the body surface area as a reference, as well as age, improves the evaluation of the degree of abnormality.34
With regard to the screening programs recommended in genetic syndromes, a high prevalence of bicuspid valve has been found in the first-degree family members of patients undergoing surgery for this condition, a finding that supports the indication to perform an echocardiogram.35
Ventricular FunctionDespite the emergence of new studies that highlight the variability between the available techniques and the need to standardize the study of myocardial deformation,36 publications on the clinical utility of investigating tissue deformation by 3D echocardiography have not been slow in coming this year. Two excellent reviews on the current status of the technique37,38 describe its potential value in the study of various cardiomyopathies. Of particular importance is its applicability to the study of left ventricular volumes and the ejection fraction, as it has shown an excellent correlation with findings obtained by cardiac magnetic resonance imaging (CMR).39 Three-dimensional echocardiography has a prominent role in the study of valve disease, in which a single parameter, the global longitudinal strain, is an independent predictor of death in patients with aortic stenosis40,41 and in those with ventricular dysfunction and mitral regurgitation.42
CardiomyopathiesImaging techniques have acquired an increasingly important role in the evaluation of drug-induced cardiotoxicity, although the choice of imaging modality and the recommended frequency of monitoring are not well defined. An interesting white paper containing recommendations on the use of the various imaging techniques43 and an excellent recent study evaluating the reproducibility of echocardiographic techniques for sequential assessment of induced myocardial dysfunction have contributed to improving the diagnosis of this disease.44 The robustness of certain predictive parameters, such as the systolic longitudinal strain, has been manifested in recent studies.45
There is growing interest in the diagnosis of morphological changes of the myocardium in individuals undergoing physical training and in those with “athlete's heart”. The specific adaptation to each type of training has generated some controversy in the literature. Recent publication of a meta-analysis that reviews the morphological changes experienced by this population has shed light on this subject.46
Heart Failure and ResynchronizationThe criteria of the American Society of Cardiology and Radiology for proper use of imaging techniques in heart failure came to light in 2013. This document expresses the efforts to critically and systematically analyze the rational use of these techniques in this condition.47
Guidelines from Europe and the United States on cardiac resynchronization,48,49 assessing the utility of imaging techniques in the study of cardiac dyssynchrony, have conferred considerable value to their use. These documents underscore the potential role of imaging techniques in predicting which patients will respond to therapy, but their use as a preliminary step before device implantation is not recommended. In a recent meta-analysis reviewing the usefulness of 3D echocardiography in resynchronization treatment, the systolic dyssynchrony index was cited as the best predictor of response.50
NUCLEAR CARDIOLOGYGuidelinesThis year witnessed the publication of the guidelines of the Society of Nuclear Medicine and Molecular Imaging, Society of Cardiovascular Computed Tomography, and American Society of Nuclear Cardiology51 on the use of hybrid, single-photon emission computed tomography (SPECT)/CT and positron emission tomography (PET)/CT systems (eg, correction of attenuation and evaluation of coronary calcifications), CT study of the coronary arteries, and acquisition of hybrid 3D images to determine myocardial viability and detect cardiac inflammation and infection.
Single-Photon Emission Computed TomographyWith regard to the prognostic evaluation of stress/rest myocardial perfusion by gated-SPECT, a study performed in Spain that included 5672 patients followed-up for more than 3 years reported that this examination, involving clinical and ergometric variables, had considerable value for predicting overall mortality and severe cardiovascular complications (cardiac death and nonfatal acute myocardial infarction).52 In addition, predictive variables of major cardiac complications and coronary revascularization have been defined in patients with normal myocardial perfusion and systolic function on SPECT.53
SPECT has also been used as a gold standard to validate angiographic scores to quantify myocardium at risk54 and as a complement in myocardial viability studies with CMR.55 Gated-SPECT analysis of motility, thickening, and ischemia are useful for categorizing segments with inconclusive results of magnetic resonance (MR) viability. There are discrepancies between SPECT and MR with regard to thickening and ischemia in up to one-third of segments defined as nonviable on MR. In acute myocardial infarction patients, an improvement in left ventricular diastolic function on myocardial perfusion gated-SPECT has been described in relation to the extent of salvaged myocardium.56
Routine follow-up with gated-SPECT has shown little value in asymptomatic patients with transposition of the great vessels corrected by arterial switch; there is a predominance of normal studies in these patients.57
Positron Emission TomographyIn a multicenter study58 including 7061 patients with suspected or known coronary disease, myocardial perfusion PET showed that evaluation of the extent and intensity of ischemia and necrosis has greater power for predicting cardiac death or any-cause mortality than conventional risk factors. In addition, the study defined the potential advantages of hybrid PET/MR systems for simultaneous evaluation of myocardial viability.59
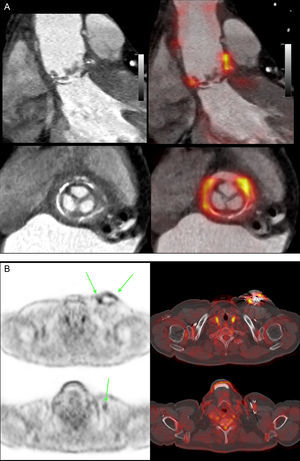
Another novel use of 18F-fluorodeoxyglucose- PET is in the evaluation of cardiac inflammation (eg, sarcoidosis) and infection60 (eg, pericarditis, myocarditis and endocarditis), particularly in patients with prosthetic valves, valved tubes, devices, and cables61 (Figures 2A and B). This capability has proven so effective that 18F-fluorodeoxyglucose uptake has been proposed as a major new criterion in the Duke diagnostic criteria of prosthetic valve endocarditis.62
A, Cardiac-synchronized computed tomography angiography slices (upper, sagittal; lower, transverse), merged with 18F-fluorodeoxyglucose positron emission tomography, in a patient with an aortic supravalvular prosthesis and a tube in the ascending aorta, showing active infection in the region around the prosthetic valve. B, positron emission tomography/computed tomography transverse slices and merging in a patient with suspected pacemaker infection. Upper image, intense uptake at the device pouch (arrows). Lower image, activity at the device cable (arrow); both findings are indicative of infection.
The year 2013 has been an extraordinary period for cardiac computed tomography (cardiac CT), with more than 100 related publications in first-line journals alone. This clearly indicates that cardiac CT is now “in fashion” for research purposes and has enormous potential in clinical practice. The simplest application is in calcium scoring. Although this test is still little used in clinical practice, accumulating evidence suggests that it is extremely useful in the assessment of cardiovascular risk and the impact of risk factors on the development of coronary disease in asymptomatic individuals,63 such that even noninvasive coronary angiography would provide little additional information in this group of patients.64
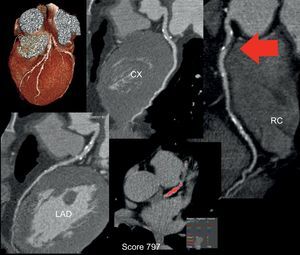
Noninvasive coronary angiography has been consolidated as a reliable technique for ruling out coronary disease and selecting patients who should be referred for conventional coronary angiography.65 One outstanding piece of news is that cardiac CT has appeared for the first time in the diagnostic algorithm of the latest guidelines (2013) of the European Society of Cardiology for the assessment of stable coronary disease,66 being considered a first-line technique in the diagnostic workup of patients at low-intermediate risk and a useful test in patients with inconclusive results in techniques for the detection of ischemia (Figure 3). In fact, current studies continue to show that emergency assessment of patients with chest pain using this technique shortens hospital stay and reduces the number of unnecessary hospital admissions.67 Two new applications of clinical interest are evaluation of the distal vessel in chronic occlusions to determine the possibility of surgical revascularization68 and demonstration that in a high percentage of patients with infarction and “normal” coronary arteries, the coronary vessels are, in reality, not so normal,69 a finding that has an evident impact on the treatment applied.
One of the active focusses of research in this field is the relationship between plaque morphology and coronary events. It has been shown that the CT “napkin-ring” sign of plaque morphology and the total volume of soft plaque are potentially useful parameters for this purpose.70 Another important focus is the possibility to perform ischemia studies with adenosine-stress cardiac CT. Validation studies of this technique vs MR ischemia studies or invasive assessment of coronary flow reserve have yielded excellent results.71 Thus, this capability will possibly be used in the clinical setting in the near future. Even more intriguing is the potential to evaluate the impact of coronary lesions on cardiac function without the need for pharmacological stress using the transluminal contrast attenuation gradient72 or noninvasive assessment of the coronary flow reserve.73
CT has become a major ally in structural interventional procedures. In transcatheter aortic valve implantation implantation, accurate prediction of the fluoroscopic angulation shortens the procedure and improves the results.74 Image resolution is unsurpassed with this technique, and cardiac CT is now appearing in clinical research with all the new prosthesis models75 and percutaneous devices.
CARDIAC MAGNETIC RESONANCEIntroductionOver the last few years, CMR has become established as the most precise technique for the characterization of myocardial tissue in both ischemic heart disease and cardiomyopathies. Furthermore, various studies have demonstrated its prognostic value in long-term follow-up.
Ischemic Heart DiseaseDespite the current controversy about determining the myocardial area at risk, CMR continues to be the technique of choice for its measurement. In a canine model, T1 and T2 mapping sequences before contrast administration had an excellent correlation with microspheres for estimating the risk area.76 Moreover, in postmyocardial infarction patients, STIR (short tau inversion recovery) sequences correlated extremely well with angiographic scores and with the area of infarcted myocardium.77 The ratio between the area at risk and the necrotic myocardium enables determination of myocardial salvage. The pain-to-reperfusion time and presence of diabetes mellitus are the main factors that have an impact on this parameter.78 In patients undergoing rescue angioplasty, there is a minimum of myocardial salvage due to the delay in opening the artery.79 To decrease the extent of necrotic tissue and increase the amount of myocardial salvage, several reperfusion strategies have been developed, such as intracoronary adenosine administration, (Spanish multicenter PROMISE study, whose results are in the publication phase), or intracoronary abciximab administration, which has not proven to be superior to the conventional intravenous route.80
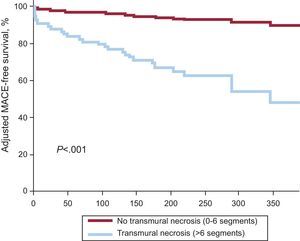
From the prognostic viewpoint, the size of necrosis is the main determinant of adverse events during follow-up.81 In this line and using a faster determination variable (quantification of the number of segments with transmural necrosis>50%), the presence of more than 6 affected transmural segments is the main predictor of events over long-term follow-up82 (Figure 4).
Adjusted survival free of major adverse cardiovascular events in patients with and without extensive transmural necrosis. MACE, major adverse cardiovascular events. Adapted with the permission of Merlos et al.82.
Various single-center83 and multicenter84 studies have demonstrated the superiority of adenosine stress CMR over SPECT for the diagnosis of coronary disease, and CMR is now established as an excellent diagnostic alternative.
CardiomyopathiesIn patients with nonischemic dilated cardiomyopathy, myocardial enhancement determines the rate of adverse events, in a manner similar to that of patients with ischemic dilated cardiomyopathy.85 This factor is independent of the ejection fraction.86
Relative to medical treatment, coronary revascularization is reported to improve the prognosis of patients with ischemic dilated cardiomyopathy, severe systolic dysfunction, and CMR evidence of viability.87 Although the study was not a clinical trial, the results demonstrate the benefit of determining myocardial viability, and they contrast with the controversial results of the STICH study.
Finally, in patients with hypertrophic cardiomyopathy who are carriers of sarcomeric mutations and have no ventricular hypertrophy, T1 mapping demonstrates an increase in extracellular fibrosis and volume. These findings indicate that fibrotic remodeling is already activated in the initial phases of disease and that prompt therapy may change the course of the condition.88
CONFLICTS OF INTERESTNone declared.