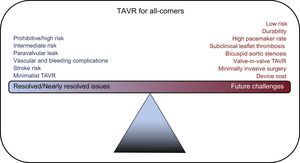
Transcatheter aortic valve replacement (TAVR) has progressed at a rapid pace driven by the Heart Valve Team approach and a rigorous commitment to evidence-based medicine. Rapid advances in technology have propelled TAVR to the forefront of treatment options. TAVR has progressed from a procedure performed in hybrid operating rooms under general anesthesia to a cathlab procedure performed under monitored anesthesia, thus reducing hospital stay and in some cases allowing “same-day” discharge. The indications for TAVR have been broadened, supported by hard evidence. Currently, TAVR is indicated for prohibitive, high and intermediate surgical risk patients. However, can TAVR become the therapy of choice for all patients in the near future? To answer this question, we need to explore some of the resolved issues and future challenges (Figure 1) that could make this technology a formidable force in the treatment of aortic stenosis (AS).
In the initial study published in 2010, TAVR was shown to be superior to medical therapy for patients with severe AS who could not undergo surgery due to prohibitive risk.1 Since then, multiple, rigorously-conducted, randomized, controlled trials have shown that TAVR was noninferior to surgical aortic valve replacement (SAVR) in patients at high and intermediate risk for surgery.2–5 In addition, a propensity-matched registry study comparing the third-generation TAVR valve (SAPIEN 3, Edwards Lifesciences, Irvine, CA) with the surgical arm of the PARTNER 2A trial in intermediate-risk patients showed that TAVR was superior to SAVR in reducing a composite endpoint of mortality, stroke, and moderate-to-severe paravalvular leak (PVL).6 This cemented the role of TAVR for patients with severe AS at prohibitive, high and intermediate risk. Since then, TAVR has been supported by guidelines as the therapy of choice in prohibitive-risk patients, and as an alternative to surgery for high- and intermediate-risk patients.
Paravalvular LeakParavalvular leak after TAVR has been shown to be associated with worse long-term cardiovascular events.2 Annular calcification and inadequate deployment were frequently quoted reasons for the occurrence of PVL. The rate of PVL is lower with SAVR as the surgeon has direct visualization with better control to prevent PVL. However, PVL after surgery remains greatly underrecognized.2,4 The rate of moderate-to-severe PVL with first- and second-generation TAVR valves (both SAPIEN and CoreValves) was high at 9% to 12%. The third-generation SAPIEN-3 valve, which has a “skirt” built around the inlet segment of the valve, reduced PVL to as low as 1.5%.1,2,4–6 The third-generation CoreValve (Evolut PRO, Medtronic plc, Dublin, Ireland), also with a skirt around the inlet segment, is showing promise in reducing PVL. The newer mechanical-expanding Lotus valve (Boston Scientific, Malborough, MA, USA) has been shown to have a very low incidence of moderate-to-severe PVL rate at 1 year (REPRISE III trial, presented at EuroPCR 2017, Paris, France). The occurrence of moderate-to-severe PVL with the newer-generation valves is significantly lower and will continue to improve.
Vascular and Major Bleeding ComplicationsThe earlier generation TAVR devices had much larger delivery systems with sheath sizes as large as 26 Fr. Current delivery systems are down to 14 Fr, and upcoming newer devices are downsizing the delivery systems to 10 Fr. Unquestionably, this technological improvement accounts for the significant decrease in vascular complications (1.2% in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy 2016 registry database) and the need to use alternative access (< 10%).7 Additionally, the systematic use of careful computed tomography angiography analysis, ultrasound-guided access, the micro-puncture needle technique, combined with the use of vascular closure devices and elimination of surgical cut-down has made large bore access and closure for TAVR extremely safe. Life-threatening bleeding complications are much lower compared with surgery and are as low as 2.4%.7 As delivery systems become slimmer and more flexible, a further reduction can be predicted in vascular and bleeding complications.
Stroke RiskEarly TAVR trials showed a 2-fold increase in stroke compared with SAVR, but with the next-generation valves, stroke rate with TAVR was similar, if not lower than with SAVR (2.7% vs 6.1%).4,6 The lower profile of the newer-generation valves along with avoidance of predilation may have helped to reduce the incidence of stroke. Although cerebral protection devices (eg, SENTINEL device, Claret Medical, Inc) were studied, routine use of these devices did not significantly reduce new lesions on magnetic resonance imaging or nondisabling stroke within 30 days.8 It remains to be seen whether they will play a role in high-risk subsets.
Minimalist TAVRApproaching the TAVR procedure in a more simplified manner from the time of procedure until “FastTrack” discharge has added benefits.9 Primarily, it improves patient satisfaction, decreases length of stay, and reduces resource use and cost. Minimalist TAVR, which is performed without general anesthesia or transesophageal echocardiography with a view to mobilizing and discharging patients early after the procedure, makes a strong economic argument favoring TAVR over SAVR. In a single-center study, minimalist TAVR cost around $10 000 less than standard TAVR with equivalent efficacy.10
FUTURE CHALLENGESLow-risk PatientsTAVR is expected to perform as well as SAVR in low-risk patients, at least in the short-term. In the NOTION trial, which randomized 280 patients at 3 Nordic centers to TAVR vs SAVR in low-risk patients (mean Society of Thoracic Surgeons Predicted Risk of Mortality score: 3.0±1.7), the primary composite endpoint of all-cause mortality, myocardial infarction, and stroke at 1 year was similar between TAVR vs SAVR (13.1% vs 16.3%, P=0.43).11 Barker et al.12 predict that in the ongoing trials studying low-risk patients, TAVR is very likely to reach a noninferiority margin compared with SAVR. They base their argument by looking at the short- and long-term mortality trends of the previous high-risk and intermediate-risk trials. In the CoreValve high-risk trial, after the similar initial procedural risk between SAVR and TAVR, SAVR was associated with higher mortality than TAVR between 1 and 4 months, subsequent to which TAVR was similar to SAVR.5 Nevertheless, in the intermediate-risk SURTAVI trial, after the initial procedural risk, TAVR was noninferior to SAVR, suggesting that high-risk patients carry an inherent mortality risk long after the procedure.4 Taking a look at these timings of cardiovascular events, Barker et al. predicted that, in the low-risk trials, mortality risk will decrease in parallel and TAVR will be noninferior to SAVR. The Ongoing PARTNER 3 trial (NCT02675114) and the CoreValve Low-Risk trial (NCT02701283) will provide much needed insight into low-risk patients.
High Pacemaker RatePermanent pacemaker (PPM) implantation rates after TAVR remain high at 10% for the SAPIEN-3, 16% for the Evolut R, and 26% for the Lotus valves. Pre-existing conduction abnormalities, calcification, valve oversizing, and procedural attributes such as depth of implantation and predilation increase the risk of PPM implants. Some of these causes can be prevented by paying attention to various aspects of the procedure. In addition, technological advances will continue to decrease the rate of PPM. For example, the next-generation Lotus valve has a depth guard, which prevents diving of the device into the left ventricular outflow tract, thereby decreasing the risk of PPM. It is crucial to avoid PPM implantation, as it is associated with increased length of stay, higher costs, a long-term risk of device infection, lead failure, and possible mortality. For TAVR to compete with SAVR, at the very least, the rate of PPM implantation should be not higher than that of SAVR.
Valve Leaflet ThrombosisSubclinical leaflet thrombosis, which can be detected on high-resolution computed tomography, occurs in both surgical and TAVR valves.13 In registry studies, the incidence of subclinical leaflet thrombosis was higher in the TAVR valves (13%) than in surgical valves (4%).14 Although no difference in the rate of stroke was seen between patients with subclinical leaflet thrombosis and those without, there was an increase in the incidence of transient ischemic attacks (4.18 per 100 person-years vs 0.6 per 100 person-years; P=.0005) in those with leaflet thrombosis.14 Anticoagulation has been shown to improve valve hemodynamics and clinical outcomes, but the advantage of the routine use of anticoagulation after TAVR for the prevention of valve leaflet thrombosis is unclear. Although there is no clear evidence of an increased rate of thromboembolic events, the mere evidence of thrombus formation on the leaflets may be an important factor for early degeneration of the valve prosthesis. Further studies are being conducted to establish the standards for diagnosing and managing valve leaflet thrombosis (ENVISAGE-TAVI-AF, NCT02943785).
Expanding TAVR Indications: Bicuspid AS and Asymptomatic ASBicuspid AS is frequently seen in patients undergoing SAVR, which usually manifests at a younger age than tricuspid AS. Bicuspid AS poses technical challenges for TAVR, especially in patients with concomitant aortopathy, and issues with valve sizing or positioning. While randomized controlled trials are lacking, in a propensity-score matched study comparing bicuspid vs tricuspid AS treated with TAVR, the newer-generation TAVR devices did not show procedural differences and were associated with similar prognosis.15 TAVR is currently not indicated for asymptomatic severe AS patients. The EARLY TAVR Trial (NCT03042104) comparing TAVR vs clinical surveillance in asymptomatic AS has started recruiting patients. Of note, the UNLOAD trial (NCT02661451) comparing TAVR vs optimal heart failure therapy in patients with moderate AS with left ventricular ejection fraction<50% is a step toward understanding the expanding role of TAVR.
DurabilityThe durability of TAVR valves remains a cardinal issue with TAVR therapy. No doubt, as the technology improves, durability will improve. However, as the indications for TAVR move toward younger patients, the durability of TAVR valves will become extremely pertinent. At 5 years, valve performance and cardiac hemodynamics were comparable to SAVR with SAPIEN valves in patients who were alive at 5 years in the PARTNER I trial.16 Similarly, the 5-year experience with the CoreValve system showed an incidence of bioprosthetic valve degeneration of 1.4%.17 It is generally accepted that the TAVR valves would last as long as the surgical bioprosthetic valves, but only time will tell.18 In addition, if valve-in-valve (ViV) TAVR can be applied safely and effectively, then the issue of durability will likely be mitigated. While there is short-term evidence to show that ViV TAVR is as good as re-do surgery, long-term results are not available.19 The current challenges with ViV TAVR include elevated postprocedural gradients, patient-prosthetic mismatch, coronary obstruction, malpositioning, ViV durability, and a higher rate of leaflet thrombosis.
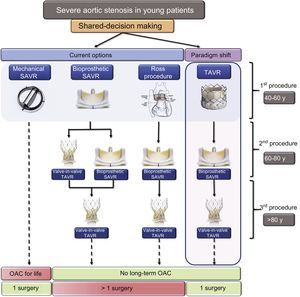
AORTIC INTERVENTION IN YOUNG PATIENTS WITH ASIn addition to the lack of evidence to support TAVR in low-risk patients, major concerns for young patients include the “durability” and “replaceability” of the aortic valves. The long-term management of severe AS in younger patients would need a paradigm shift from the current model based on surgical risk to a new algorithm based on age or therapeutic options (Figure 2). The goal is to avoid long-term anticoagulation and more than one open-heart surgery during the patient's lifetime. Patients aged 40 to 60 years with severe AS would have a variety of choices with application of shared-decision making principles in choosing the valve. For example, a 50-year-old patient with severe AS would have 2 to 3 choices. The current options include the following: a) an open-heart surgery procedure with a mechanical aortic valve that will last the patient's lifetime, but with the downside of life-long anticoagulation: b) open-heart surgery with a bioprosthetic aortic valve, but when the valve degenerates, ViV TAVR or re-do surgery will be necessary as the potential subsequent options; and c) a Ross procedure (autologous pulmonary valve to replace a diseased aortic valve) is a known viable long-term option for adults with severe AS, but homograft failure and autograft dysfunction are significant impediments.20 While the downside of the first option is the need for life-long oral anticoagulation, the other 2 options may require more than 1 open-heart surgical procedure. We propose a paradigm shift in shared-decision making by introducing TAVR as the first option for younger patients. In this case, TAVR may last approximately 10 years until patients are aged 60 to 80 years, when they could undergo open-heart surgery with a surgical bioprosthetic aortic valve. If bioprosthetic valve dysfunction subsequently occurs when the patient is aged > 80 years, ViV TAVR can be performed, thus eliminating the need for long-term anticoagulation. The advantage is that, with a single open-heart surgical procedure, AS in young patients can be managed without the need for long-term anticoagulation.
Proposed algorithm for initial and subsequent procedure selection in younger patients with severe aortic stenosis. Patients who opt for a mechanical aortic valve would need long-term anticoagulation. For those who would like to avoid long-term anticoagulation, an initial bioprosthetic SAVR or Ross procedure are viable options. A paradigm shift in choosing TAVR as the initial choice has the advantage of avoiding long-term anticoagulation with a single open-heart surgical procedure for the patient's lifetime. OAC, oral anticoagulants; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
TAVR is set to become a prodigious therapy, if not for all patients then for most patients with severe AS. Technological advances in TAVR devices, significant improvement in outcomes and perhaps even more importantly, patient preference for less invasive therapies will make TAVR the preferred therapy for patients with severe AS. However, there will remain a certain subset of patients in whom SAVR may be the preferred option, such as those requiring aortic root enlargement, young patients preferring mechanical valves, concomitant significant coronary artery disease and other valvular heart disease. Advances in minimally-invasive surgery have significantly shortened cardiopulmonary bypass time and length of stay. It remains to be seen whether minimally invasive surgery with a sutureless aortic valve will provide advantages over TAVR.
The current high cost of TAVR therapy has huge implications in the dissemination of this option. However, in appropriately-selected patients who undergo minimalist TAVR with a short length of stay, medical costs are lower and may be even be cost-effective compared with SAVR.21 More studies are needed to determine the long-term economic impact of this technology. Like any new technology, TAVR is likely to become more widely adopted with industry participation, which will eventually reduce costs.
In conclusion, TAVR is not yet ready for all-comers with severe AS. Nonetheless, it is on the way to becoming the major player in treating most patients with severe AS. A paradigm shift away from the current surgical-risk based model to novel models in younger patients is needed. There will continue to be a few anatomical limitations that will still require open-heart surgery. The resolution of future challenges with TAVR will likely be driven by patient preference for less invasive procedures and rapid strides in technological development. Moreover, the medical cost of TAVR therapy needs to be reduced to make this technology economically feasible for hospitals and global health care.
CONFLICTS OF INTERESTC.E. Ruiz is an investigator (unpaid) for the Portico Trial. Institutional educational grants were received from Medtronic and Abbott-St Jude Medical. T.K.R. Pasala has no conflict of interest.
.