To assess the potential association between recipient Toxoplasma gondii serostatus and outcomes after heart transplant (HT).
MethodsWe conducted a retrospective single-center study of 657 HT recipients from 1991 to 2015. Survival and the incidence of adverse clinical events of T. gondii-seropositive (n = 481) vs T. gondii-seronegative (n = 176) recipients were compared by means of 2 different multivariable Cox regression models. Model 1 included solely age and sex, and model 2 included other potential confounders.
ResultsOver a median follow-up of 2903 days (interquartile range: 898-4757), 250 seropositive recipients (52%) and 72 seronegative recipients (41%) died. Univariable analysis showed increased posttransplant mortality among T. gondii-seropositive recipients (hazard ratio [HR] = 1.31; 95% confidence interval [95%CI], 1,00-1.70). After multivariable adjustment, the statistical significance of this association was lost (model 1: HR = 1.09; 95%CI, 0.83-1.43; model 2:HR = 1.12; 95%CI, 0.85-1.47). Recipient T. gondii seropositivity was independently associated with an increased risk of acute rejection (model 1: HR = 1.36; 95%CI, 1.06-1.74; model 2: HR = 1.29; 95%CI, 1.01-1.66). Multivariable models showed no statistically significant impact of recipient T. gondii serostatus on the incidence of infection, malignancy, coronary allograft vasculopathy, or the composite outcome of cardiac death or retransplant. No significant association was found between donor-recipient T. gondii serostatus matching and posttransplant outcome.
ConclusionsIn this study, recipient T. gondii serostatus was not an independent predictor of long-term post-HT outcome.
Keywords
Heart transplant (HT) improves survival and quality of life in patients with refractory heart failure and no absolute contraindications.1 To optimize the screening process, it is important to understand the factors that increase the risk of mortality after HT.
The question of whether or not preoperative Toxoplasma gondii serostatus is an independent prognostic factor in HT recipients continues to be a topic of debate.2 Some years ago, an early observational single-center study3 showed a significant drop in post-HT survival in T. gondii-seropositive recipients compared with seronegative recipients; this effect seemed to be attributable to a higher incidence of graft vascular disease and a higher risk of death due to this complication. However, 2 subsequent single-center studies4,5 were unable to confirm the previous finding.3 None of the 3 studies published to date has found a significant influence of donor T. gondii serostatus or of donor-recipient serostatus matching on post-HT prognosis.3–5
Because the controversy persists, the aim of this study was to evaluate the potential impact of T. gondii serostatus of HT recipients on long-term prognosis after this surgery.
METHODSStudy DescriptionAn observational retrospective study was conducted with a historical cohort of HT recipients in the Complejo Hospitalario Universitario A Coruña from the start of the program in April 1991 until February 2015. The information for the study was obtained from a local database. For the analysis, patients were only selected if their pre-HT T. gondii serostatus was known.
At the hospital, the T. gondii serostatus is currently tested by immunoassay (Elecsys Toxo IgG, Roche Diagnostics) and the result is considered positive if the T. gondii immunoglobulin G (IgG) concentration is > 3 IU/mL. In the past, other commercial immunoassay kits were used to detect specific IgGs with lower sensitivity. The IgG avidity assays are not available at the hospital at present.
The baseline characteristics and post-HT outcome of seropositive recipients were compared with those of T. gondii-seronegative patients. The study protocol was approved by the Autonomous Clinic Research Ethics Committee of Galicia.
Clinical ProtocolAll patients were treated and monitored in accordance with the hospital protocol. During the postoperative period, induction therapy consisted of muromonab-CD3 or basiliximab. The maintenance immunosuppressive regimen consisted of different combinations of steroids, calcineurin inhibitors (cyclosporin or tacrolimus), antiproliferative agents (azathioprine or mycophenolate mofetil), and/or mTOR inhibitors (sirolimus or everolimus).
Regular endomyocardial biopsies were performed during the first year after HT and afterwards only if there was clinical suspicion of acute rejection. Stable patients attended clinic visits, which included an electrocardiogram and echocardiogram every 3 to 6 months after the first year post-HT. Coronary angiographies were performed for routine screening of graft vascular disease in patients with clinical suspicion (and also in asymptomatic patients as of 2003) at 1 month, 1 year, 5 years, and 10 years post-HT.
At the hospital, all T. gondii-seronegative recipients who receive a cardiac graft from a seropositive donor or with unknown serostatus are treated with oral pyrimethamine (25 mg daily) and folinic acid (15 mg daily) solution for the first 6 months post-HT. Additionally, all HT recipients receive Pneumocystis jiroveci chemoprophylaxis with oral sulfamethoxazole-trimethoprim (800/160 mg daily) for at least the first 12 months post-HT.
OutcomesStudy patients were followed up from the time of HT until death or a new HT; otherwise, follow-up was closed on 30 April 2015. The main study outcome was total post-HT mortality. Other outcomes analyzed were combined cardiac death or cardiac retransplant, acute rejection, graft vascular disease, infection, and neoplasms.
The causes of death were collected from autopsy reports or medical death certificates. Cardiac death was defined as any death caused by heart failure, myocardial ischemia, or arrhythmia, including those caused by primary graft dysfunction, acute rejection, or graft vascular disease, as well as any unexplained sudden deaths. The outcome of neoplasm included any solid, cutaneous, or lymphoid malignant neoplasms. The outcome of infection was defined as any microbiologically proven or suspected infection requiring hospitalization and/or intravenous antibiotic therapy. The outcome of acute rejection was defined as any episode of acute cellular rejection of grade ≥ 2R,6 whether symptomatic or not, or an episode of acute humoral rejection of pAMR (antibody-mediated rejection) grade ≥ 1,7 accompanied by clinical symptoms of heart failure and/or systolic dysfunction of the graft, or any episode of acute rejection that was suspected but not biopsy-proven that would have needed treatment with intravenous steroid bolus, plasma exchange, intravenous immunoglobulins, or thymoglobulin. Graft vascular disease was defined as the presence of stenosis ≥ 50% of the luminal diameter of any of the 3 main epicardial coronary vessels or their branches visualized on coronary angiography or postmortem evidence (only if considered to be the main cause of death according to the autopsy findings).
Statistical AnalysisIn this study, qualitative variables are expressed as mean ± standard deviation or median (interquartile range), according to the normality of their distribution, and categorical variables are expressed as proportions. The baseline characteristics of T. gondii-seropositive and -seronegative patients were compared using chi-square tests, the Student t test, or Mann-Whitney U test as applicable.
The effect of recipient T. gondii serostatus on each outcome analyzed was adjusted by 2 multivariable Cox regression models. Model 1 included recipient age and sex as well as recipient T. gondii serostatus. Model 2 included the same variables as model 1 along with variables showing an independent statistical association with the outcome analyzed (P < .05), as well as other variables with an asymmetric distribution between the 2 study groups that were considered potential confounding factors, based on clinical experience and the literature. Model 2 was also used to investigate a potential influence of donor T. gondii serostatus on mortality after HT. Statistical significance was set at P < .05. The statistical analysis was performed with SPSS 20.
RESULTSStudy PopulationAt the hospital, 703 patients underwent HT between April 1991 and February 2015. Recipient T. gondii serostatus before HT was known in 657 (93%) patients and was positive in 481 (73%). A total of 46 (7%) recipients with unknown T. gondii serostatus were excluded from the analysis.
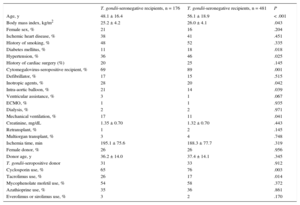
A comparison of the baseline clinical characteristics of the recipients based on their T. gondii serostatus is shown in Table 1. Seropositive patients were older and had a higher body mass index and higher prevalence of diabetes and cytomegalovirus seropositivity, whereas the use of preoperative life support measures was more common in seronegative patients. Furthermore, a higher percentage of seronegative patients received initial therapy with tacrolimus.
Baseline Clinical Characteristics of the Study Patients
| T. gondii-seronegative recipients, n = 176 | T. gondii-seronegative recipients, n = 481 | P | |
|---|---|---|---|
| Age, y | 48.1 ± 16.4 | 56.1 ± 18.9 | < .001 |
| Body mass index, kg/m2 | 25.2 ± 4.2 | 26.0 ± 4.1 | .043 |
| Female sex, % | 21 | 16 | .204 |
| Ischemic heart disease, % | 38 | 41 | .451 |
| History of smoking, % | 48 | 52 | .335 |
| Diabetes mellitus, % | 11 | 18 | .018 |
| Hypertension, % | 36 | 46 | .025 |
| History of cardiac surgery (%) | 20 | 25 | .145 |
| Cytomegalovirus-seropositive recipient, % | 69 | 89 | .001 |
| Defibrillator, % | 17 | 15 | .515 |
| Inotropic agents, % | 28 | 20 | .042 |
| Intra-aortic balloon, % | 21 | 14 | .039 |
| Ventricular assistance, % | 3 | 1 | .067 |
| ECMO, % | 1 | 1 | .935 |
| Dialysis, % | 2 | 2 | .971 |
| Mechanical ventilation, % | 17 | 11 | .041 |
| Creatinine, mg/dL | 1.35 ± 0.70 | 1.32 ± 0.70 | .443 |
| Retransplant, % | 1 | 2 | .145 |
| Multiorgan transplant, % | 3 | 4 | .748 |
| Ischemia time, min | 195.1 ± 75.6 | 188.3 ± 77.7 | .319 |
| Female donor, % | 26 | 26 | .956 |
| Donor age, y | 36.2 ± 14.0 | 37.4 ± 14.1 | .345 |
| T. gondii-seropositive donor | 31 | 33 | .912 |
| Cyclosporin use, % | 65 | 76 | .003 |
| Tacrolimus use, % | 26 | 17 | .014 |
| Mycophenolate mofetil use, % | 54 | 58 | .372 |
| Azathioprine use, % | 35 | 36 | .861 |
| Everolimus or sirolimus use, % | 3 | 2 | .170 |
ECMO, extracorporeal membrane oxygenation.
Data are expressed as percentage or mean ± standard deviation.
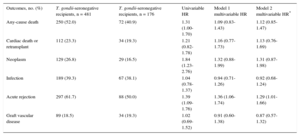
Patients were followed up for a mean of 2903 days (interquartile range, 898-4757) after HT. During this period, 322 (49%) patients died and 9 (1%) received a second HT. Death was of cardiac origin in 137 (41%) patients and was noncardiac in 185 (59%). The distribution of the causes of death according to T. gondii serostatus is shown in Table 2.
Cause of Death in Toxoplasma gondii-seropositive and -seronegative Recipients
| T. gondii-seronegative recipients, n = 72 | T. gondii-seronegative recipients, n = 250 | |
|---|---|---|
| Cardiac death, % | 33 (46) | 104 (42) |
| Primary graft failure (early) | 13 | 23 |
| Acute rejection | 5 | 12 |
| Graft vascular disease | 8 | 29 |
| Sudden death | 7 | 33 |
| Nonspecific graft failure (late) | 0 | 7 |
| Noncardiac cause, % | 39 (54) | 146 (58) |
| Infection | 6 | 47 |
| Neoplasm | 8 | 53 |
| Vascular disease | 8 | 9 |
| Unspecified multiorgan failure | 5 | 8 |
| Other | 12 | 29 |
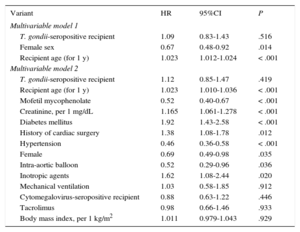
Total nonadjusted mortality was significantly higher in T. gondii-seropositive patients than in seronegative patients (univariable hazard ratio [HR] = 1.31; 95% confidence interval [95%CI], 1.00-1.70). However, this effect lost its statistical significance after the multivariable adjustment. The HR of total mortality, adjusted for recipient age and sex (multivariable model 1), was 1.09 (95%CI, 0.83-1.43; Table 3). No relevant changes were observed in this association when the adjustment included other potential confounding factors (multivariable model 2: HR = 1.12; 95%CI, 0.85-1.47; Table 3). The multivariable Cox regression models used for total mortality are shown in Table 4.
Summary of Study Outcomes
| Outcomes, no. (%) | T. gondii-seronegative recipients, n = 481 | T. gondii-seronegative recipients, n = 176 | Univariable HR | Model 1 multivariable HR | Model 2 multivariable HR* |
|---|---|---|---|---|---|
| Any-cause death | 250 (52.0) | 72 (40.9) | 1.31 (1.00-1.70) | 1.09 (0.83-1.43) | 1.12 (0.85-1.47) |
| Cardiac death or retransplant | 112 (23.3) | 34 (19.3) | 1.21 (0.82-1.78) | 1.16 (0.77-1.73) | 1.13 (0.76-1.69) |
| Neoplasm | 129 (26.8) | 29 (16.5) | 1.84 (1.23-2.76) | 1.32 (0.88-1.99) | 1.31 (0.87-1.98) |
| Infection | 189 (39.3) | 67 (38.1) | 1.04 (0.78-1.37) | 0.94 (0.71-1.26) | 0.92 (0.68-1.24) |
| Acute rejection | 297 (61.7) | 88 (50.0) | 1.39 (1.09-1.76) | 1.36 (1.06-1.74) | 1.29 (1.01-1.66) |
| Graft vascular disease | 89 (18.5) | 34 (19.3) | 1.02 (0.69-1.52) | 0.91 (0.60-1.38) | 0.87 (0.57-1.32) |
HR, hazard ratio.
The HR estimation assumes that T. gondii-seronegative patients are the reference category.
Covariables included in the models:
Any-cause death: recipient T. gondii serostatus, diabetes, hypertension, recipient sex, recipient age, history of cardiac surgery, creatinine, recipient cytomegalovirus serostatus, body mass index, balloon pump, inotropic agents, mechanical ventilation, mycophenolate mofetil, and tacrolimus.
Cardiac death or retransplant: recipient T. gondii serostatus, diabetes, hypertension, recipient sex, recipient age, recipient cytomegalovirus serostatus, body mass index, balloon pump, defibrillator, inotropic agents, mechanical ventilation, mycophenolate mofetil, and tacrolimus.
Neoplasm: recipient T. gondii serostatus, recipient sex, recipient age, smoking, recipient cytomegalovirus serostatus, body mass index, and tacrolimus.
Infection: recipient T. gondii serostatus, diabetes, hypertension, recipient sex, recipient age, donor age, recipient cytomegalovirus serostatus, body mass index, balloon pump, inotropic agents, mechanical ventilation, mycophenolate mofetil, and tacrolimus.
Acute rejection: recipient T. gondii serostatus, recipient sex, recipient age, smoking, multiorgan transplant, recipient cytomegalovirus serostatus, body mass index, mycophenolate mofetil, and tacrolimus.
Graft vascular disease: recipient T. gondii serostatus, diabetes, hypertension, recipient sex, recipient age, donor age, recipient cytomegalovirus serostatus, body mass index, and tacrolimus.
Survival Analysis in Heart Transplant Recipients: Multivariable Cox Regression Models
| Variant | HR | 95%CI | P |
|---|---|---|---|
| Multivariable model 1 | |||
| T. gondii-seropositive recipient | 1.09 | 0.83-1.43 | .516 |
| Female sex | 0.67 | 0.48-0.92 | .014 |
| Recipient age (for 1 y) | 1.023 | 1.012-1.024 | < .001 |
| Multivariable model 2 | |||
| T. gondii-seropositive recipient | 1.12 | 0.85-1.47 | .419 |
| Recipient age (for 1 y) | 1.023 | 1.010-1.036 | < .001 |
| Mofetil mycophenolate | 0.52 | 0.40-0.67 | < .001 |
| Creatinine, per 1 mg/dL | 1.165 | 1.061-1.278 | < .001 |
| Diabetes mellitus | 1.92 | 1.43-2.58 | < .001 |
| History of cardiac surgery | 1.38 | 1.08-1.78 | .012 |
| Hypertension | 0.46 | 0.36-0.58 | < .001 |
| Female | 0.69 | 0.49-0.98 | .035 |
| Intra-aortic balloon | 0.52 | 0.29-0.96 | .036 |
| Inotropic agents | 1.62 | 1.08-2.44 | .020 |
| Mechanical ventilation | 1.03 | 0.58-1.85 | .912 |
| Cytomegalovirus-seropositive recipient | 0.88 | 0.63-1.22 | .446 |
| Tacrolimus | 0.98 | 0.66-1.46 | .933 |
| Body mass index, per 1 kg/m2 | 1.011 | 0.979-1.043 | .929 |
95%CI, 95% confidence interval; HR, hazard ratio.
No statistically significant differences were observed between T. gondii-seropositive and -seronegative recipients in the cumulative incidence of the combined outcome of cardiac death or retransplant, or in the univariable analysis (log rank P = .331; HR = 1.21; 95%CI, 0.82-1.78), or in the multivariable models (model 1: HR = 1.16; 95%CI, 0.77-1.73; model 2: HR = 1.13; 95%CI, 0.76-1.69; Table 3).
The univariable analysis revealed a statistically significant increase in the cumulative incidence of acute rejection in T. gondii-seropositive patients (log rank P = .007; HR = 1.39; 95%CI, 1.09-1.76), which was maintained in the multivariable analyses (model 1: HR = 1.36; 95%CI, 1.06-1.74; model 2: HR = 1.29; 95%CI, 1.01-1.66).
The univariable analysis also showed an increase in the risk of neoplasms in T. gondii-seropositive patients (log rank P = .003; HR = 1.84; 95%CI, 1.23-2.76). However, this association was not statistically significant after the multivariable adjustment (model 1: HR = 1.32; 95%CI, 0.88-1.99; model 2: HR = 1.31; 95%CI, 0.87-1.98).
No statistically significant differences in T. gondii serostatus were observed in the cumulative incidence of graft vascular disease (log rank P = .920; univariable HR = 1.02; 95%CI, 0.69-1.52), infections (log rank P = .808; univariable HR = 1.04; 95%CI, 0.78-1.37), the univariable analysis, or in the various multivariable models.
Donor-recipient Matching of Toxoplasma gondii SerostatusT. gondii serostatus of the heart donor was known in 441 (67%) transplants and was positive in 323 (73%) of them. The distribution of T. gondii donor and recipient serostatus is shown in Table 1.
The use of seropositive donors in seronegative recipients showed a tendency toward a better post-HT outcome than other combinations. However, the univariable analysis did not reveal a statistically significant difference between the groups in total mortality (P = .075 in the global comparison).
When the donor (–)/recipient (–) combination was taken as the reference category, the multivariable HRs of total mortality post-HT were 0.71 (95%CI, 0.39-1.30) for the donor (+)/recipient (–) combination, 1.05 (95%CI, 0.67-1.63) for the donor (–)/recipient (+) combination, and 1.22 (95%CI, 0.79-1.89) for the donor (+)/recipient (+) combination.
DISCUSSIONIn this single-center cohort of 657 HT recipients who were followed up for a mean of more than 8 years after surgery, T. gondii-seropositive patients showed moderately lower nonadjusted survival than seronegative patients. The association was no longer statistically significant after the multivariable adjustment for demographic factors (age and sex); other regression models that included a broader spectrum of covariables were also unable to show an independent effect of T. gondii serostatus on post-HT survival. These results suggest that the association between the 2 variables is strongly conditioned by a confounding bias derived from significant differences in the baseline clinical characteristics of the groups compared. In this series, T. gondii-seropositive patients had a higher mean age8 and a higher prevalence of adverse clinical conditions, such as diabetes mellitus9 or cytomegalovirus seropositivity.10 In addition, the use of tacrolimus11 was more common among seronegative patients.
Arora et al.3 were the first to suggest the hypothesis that T. gondii seropositivity could be an adverse prognostic factor in HT recipients. In a series of 288 patients who received HT at a single center between 1994 and 2005 and who were followed up for a mean of 5.5 years, these authors described a statistically significant and independent association between T. gondii seropositivity and a higher risk of late mortality (> 1 year) after the surgery (multivariable HR = 1.9). This result was attributed to the higher incidence of graft vascular disease (multivariable HR = 2.7) and death due to this condition (multivariable HR = 4.4). To explain their findings, the authors propose a pathophysiologic hypothesis, not yet confirmed, according to which chronic T. gondii infection would contribute to the development of graft vascular disease by stimulating various immunologic responses.
Some authors12 questioned the analysis performed by Arora et al.3 due to concerns about the statistical methodology used to control for potential confounding biases. Two subsequent single-center analyses4,5 were also unable to reproduce its results. In contrast with the initial study, Doesch et al.5 described a series of 344 patients who received HT between 1989 and 2008 and who were followed up for a mean of 5.7 years; in these patients, survival was higher among T. gondii-seropositive individuals. In another single-center cohort of 582 patients who received a transplant between 1984 and 2011 and who were followed up for a mean of 8.3 years, Van Hellemond et al.4 observed an increased unadjusted mortality in T. gondii-seropositive recipients. However, similar to the findings observed in these analyses, this association lost its statistical significance after the multivariable adjustment. As in this series, seropositive patients in the cohort studied by Van Hellemond et al.4 were older on average and had a higher prevalence of adverse comorbidities than seronegative patients.
It could be argued that the inability of multivariable models to detect an independent effect of T. gondii seropositivity on post-HT survival is due to insufficient statistical power. In the authors' opinion, this hypothesis is highly unlikely, considering that the sample size of this series, the number of events, and the duration of follow-up are higher than in the study conducted by Arora et al.3 Furthermore, the strength of the statistical association is low–the multivariable HRs vary from 1.09 to 1.12, according to the model selected–, which does not support a causal relationship. However, the discrepancies between our results and those of other earlier studies3,5 should be interpreted in the context of significant heterogeneity among the study populations, a variable prevalence of T. gondii seropositivity, and intercenter variations in chemoprophylaxis regimens.
This series did not observe a significant effect in T. gondii serostatus on the incidence of graft vascular disease or cardiac death. This finding is consistent with the 2 most recent studies4,5 but, as mentioned earlier, is in contrast with the initial study.3 Nevertheless, a moderately increased risk of acute rejection has been observed among T. gondii-seropositive patients. Chronic T. gondii infection is known to be a proinflammatory stimulus that increases interleukin-12 and interferon-gamma production by the host's immune system13 and, therefore, could hypothetically be a trigger for rejection. Irrespective of this possibility, this moderately increased incidence of acute rejection did not produce a significant increase in mortality; moreover, acute rejection was a rare cause of death in this series and caused only 5% of all the deaths recorded. This paradoxical finding could be attributed to the fact that most rejection episodes were seen in protocol biopsies before rejection caused symptoms or graft dysfunction and were still treated aggressively.
The univariable analysis revealed an increased unadjusted incidence of neoplasms after HT among T. gondii-seropositive patients. As in the case of total mortality, the statistical significance of this association disappeared after multivariable adjustment, thus suggesting that this would again be attributable to a confounding bias derived from an asymmetric distribution of risk factors for neoplasms (particularly age) between the groups. To date, there is no evidence that T. gondii infection and/or chemoprophylaxis against this microorganism are potential carcinogens.
This analysis also failed to show a significant impact of donor-recipient matching with regard to T. gondii serostatus on post-HT survival. This result should be interpreted cautiously because almost a third of patients with unknown donor serostatus were excluded, but the result is consistent with those of previous studies,3–5 even when taking this limitation into account. The lack of a negative prognostic effect of implanting a graft from a T. gondii-seropositive donor in a seronegative recipient has been mentioned as an argument against a hypothetical influence of T. gondii serostatus on post-HT outcome.2
Limitations and StrengthsThis study has several limitations. Because of the retrospective design, the series could be subject to inherent selection and information biases for this type of investigation. In addition, because this was a single-center study, its results may not be directly extrapolable to other populations. A comprehensive multivariable analysis was performed, and an effort was made to control potential confounding biases. However, it cannot be ruled out that other untested covariables may have influenced the statistical associations observed. The validity of the analysis of the prognostic impact of recipient T. gondii serostatus is limited because these data are unknown in a third of the individuals studied. Additionally, the lack of information on actual completion of chemoprophylaxis against T. gondii has made it difficult to draw conclusions. Last, the protocol of this center does not contemplate the performance of serial serologic determinations or DNA screening tests to monitor T. gondii infection in transplant recipients; for this reason, it was impossible to analyze the incidence of seroconversion or first-time T. gondii infection after HT or to establish a correlation between this type of infection and acute rejection episodes or changes in immunosuppressive therapy.
The strengths of this study over similar, previously-conducted investigations include a larger sample size and longer follow-up. The study also has the added value of confirmation of findings obtained in other populations in this setting, with implications for the strength of arguments refuting the hypothesis of an independent prognostic effect of T. gondii serology on the prognosis of HT recipients.
CONCLUSIONSIn this study, T. gondii-seropositive HT recipients had lower nonadjusted survival than seronegative recipients. More than a true causal association, these results seem attributable to a confounding bias resulting from a higher mean age at the time of HT and a higher prevalence of associated comorbidities; indeed, 2 different multivariable models failed to show an independent effect of recipient T. gondii serostatus on post-HT survival. In short, there does not appear to be solid scientific evidence to recommend a major change in the therapeutic approach currently adopted in T. gondii-seropositive HT recipients. However, in view of the discrepancy in the results of different single-center series, analysis of multicenter registries may be of interest for resolving the controversy concerning this issue.
- -
The literature reveals considerable controversy regarding the potential prognostic impact of T. gondii serostatus in HT recipients. This hypothesis is supported by a small single-center study, but its results could not be reproduced in 2 subsequent studies. In addition, although various pathophysiologic hypotheses have been proposed and consider chronic T. gondii infection to play a causative role in the genesis of graft vascular disease, there is no solid evidence to support them.
- -
This work does not support an independent prognostic impact of T. gondii serostatus in HT recipients. Although long-term unadjusted mortality was significantly lower in seropositive recipients, this effect lost statistical significance after multivariable adjustment. These findings suggest that the association between T. gondii serostatus and the prognosis of HT recipients might not be a true causal relationship, but rather the result of a confounding bias derived from a higher mean age at the time of surgery and a higher prevalence of associated comorbidities.
None declared.
The authors would like to thank Zulaika Grille-Cancela, Paula Blanco-Canosa, Cristina Costa-Graña, Carmen Naya-Leira, and Pilar Fariñas-Garrido for their assistance with the study.