Nonfluoroscopic navigation systems were first reported 20 years ago and started to be used in daily clinical practice for cardiac electrophysiology in 1998.1 As with many technologies, after a phase of excessive initial optimism, one of skepticism rapidly took hold. The usefulness of these systems was called into question and they were even compared with toys (the tools or toys dilemma).2 However, in the following years, new systems appeared3 and improvements, both in hardware and software, were continuously implemented. After the initial reticence subsided, these systems are now an essential component in modern cardiac catheterization laboratories and the range of arrhythmic substrates treated is becoming ever broader.4Revista Española de Cardiología has recently published 2 interesting articles on the use of these systems.5,6
One of these studies, by Ballesteros et al.,5 reports the first results in Spain of a new nonfluoroscopic navigation system, the Rhythmia system manufactured by Boston Scientific. The authors describe the largest series published to date on ablation of atrial fibrillation (AF) guided by this system. The use of this system in their prospective series was compared with historical cohorts, in which other conventional electroanatomic navigation systems for AF ablation had been used.7,8 In all cases, the historical outcomes were comparable with the outcomes reported with the Rhythmia system in terms of effectiveness and safety. The total procedure time was similar or longer and the fluoroscopy time was longer in the historical cohorts than with the Rhythmia system. Like this study, these previous studies used for comparison lacked an analysis of hard endpoints, not just of mortality but also arrhythmia-free follow-up and improvement in quality-of-life endpoints. Nevertheless, this shortcoming does not negate the value of this study, which should be interpreted as the presentation of initial findings with a novel technique. It is likely that the operators are still at the beginning of the learning curve inherent in any new technology.
However, we should ask what the system presented here really adds to those already on the market. First, it should be remembered that this study, like previous publications, compares this new navigation system with older versions of the habitual navigation systems, and that new versions are now available for both the Carto system (Biosense-Webster) and the Ensite system (Abbott). These new versions enable automatic generation of high-density maps (Table and Video of the supplementary material).9 This reflects the accelerated technological development that has occurred in the field of electrophysiology in recent years. Electrophysiology went from having little more than fluoroscopy, electrocardiography, and pacemakers to having a plethora of systems such as cryoablation and laser ablation techniques, intracardiac echocardiography, remote navigation robots, rotor mapping systems, contact sensors, and esophageal temperature probes, among others. These systems require medical training and also trained laboratory personnel, as well as connectors and cables that complicate the daily routine and have even led to some of these systems being uninstalled after a few years. Therefore, before new technologies are adopted, questions such as the value that they provide beyond what is already available and at what cost, not just economic, should be addressed. More than ever, the old aphorism tools or toys should be taken into consideration.
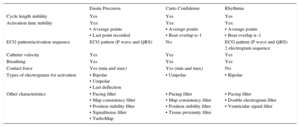
Main Filters for Obtaining High-Density Activation Maps in the Most Recent Versions of the Current Electroanatomical Navigation Systems
| Ensite Precision | Carto Confidense | Rhythmia | |
|---|---|---|---|
| Cycle length stability | Yes | Yes | Yes |
| Activation time stability | Yes: • Average points • Last point recorded | Yes: • Average points • Beat overlap n–1 | Yes: • Average points • Beat overlap n–1 |
| ECG pattern/activation sequence | ECG pattern (P wave and QRS) | No | ECG pattern (P wave and QRS) 2 electrogram sequence |
| Catheter velocity | Yes | Yes | Yes |
| Breathing | Yes | Yes | Yes |
| Contact force | Yes (min and max) | Yes (min and max) | No |
| Types of electrograms for activation | • Bipolar • Unipolar • Last deflection | • Unipolar | • Bipolar |
| Other characteristics | • Pacing filter • Map consistency filter • Position stability filter • Signal/noise filter • TurboMap | • Pacing filter • Map consistency filter • Position stability filter • Tissue proximity filter | • Pacing filter • Double electrogram filter • Ventricular signal filter |
We should also remember that the system presented is one of the earliest versions. On the one hand, this has the advantage that improvements may be available in successive versions. On the other, however, it means that many of the options already available for other systems will take time before being added to this one. Improvements include showing the values of contact force between the ablation catheter and cardiac tissue on the navigation map itself. This is quickly becoming standard clinical practice. Other improvements may appear of little importance at first glance, but in daily practice may facilitate the use of the system and reduce demands on laboratory staff in particular.
Even if such considerations are repeated ad nauseam, in a scenario of limited resources that are increasingly controlled and assessed, it is also important to analyze the increase in cost associated with a new technology for a given individual procedure. Above all, these costs should be balanced against the possible benefits of the use of the new technology. The cost analysis should take into account 3 aspects. The first is the initial investment to acquire the system and train staff in its use. The second is the cost of consumables, whether these correspond to the special catheters that are required, as is the case of the Orion catheter for the Rhythmia system, or dedicated ablation catheters. In this study, in addition to the Orion catheter, an additional guided vascular access sheath was used with the system, which could have a significant impact on the costs of the procedure. A third aspect of cost is derived from the additional time that may be required to use the new technology. For example, the patient may need to be prepared to perform the procedure. At least in the study in question, this does not appear to be longer, although this observation should be interpreted taking into account the limitations of a nonrandomized design.
Finally, one of the most important results of this study is that the Rhythmia system appears to better identify the gaps in persistence of conduction in the case of reconnection of pulmonary veins when compared with the old version of one of the classic navigation systems. However, this finding may be of little practical relevance in AF ablation, given that the disconnection of the reconnected pulmonary veins is a simple procedure, which is successful in almost all cases and often does not even require the support of a navigation system, or at least does not justify use of the new system. On this point, we should mention that disconnection of the pulmonary veins for AF ablation is not the procedure that most stands to benefit from this system, given that it is a highly anatomical procedure that does not currently require electroanatomical activation or voltage maps, the strong point of this new system. What is important is to know the geometry of the left atrium and the pulmonary veins as accurately as possible, to have good stability for the spatial position of the geometry during the procedure and, above all, accurate representation of the ablation catheter on the surface of the cardiac geometry. The classic navigation systems that have been years in development seem stronger in these aspects given the improvements over the years. Given that the main strength of the system is mapping of macroreentrant arrhythmias, it might be more interesting to study the behaviour of this navigation system in the mapping and ablation of such arrhythmias. However, these tachycardias, particularly macroreentrant atrial tachycardias, currently represent a minority of tachycardias seen in the catheterization laboratory. Most interventions are for AF, common atrial flutter, and supraventricular tachycardias with other underlying mechanisms. The benefit of having this new navigation system could be brought into question when other systems can be used efficiently for these procedures, with no additional costs for consumables or complications in daily practice. Nevertheless, this study shows that the system seems to be able to detect narrow isthmuses of residual conduction better than convention for point-by-point mapping. These isthmuses are represented as gaps in the pulmonary veins. In this respect, we should congratulate the authors as, to date, no other study has demonstrated this, and the use of a reconnection model of the pulmonary veins for this purpose is valid and imaginative.
The study by Álvarez et al.,6 also published in Revista Española de Cardiología, shows that guided procedures can be performed exclusively using a nonfluoroscopic navigation system, thereby avoiding the use of ionizing radiation. The development of this idea was pioneered in Spain,10 although it was received with certain skepticism to begin with. Fluoroscopy is a technology dating from the 19th century and has been used ever since to guide medical procedures. However, its use is known to be associated with substantial risks from ionizing radiation11 and orthopedic risks associated with the use of radiological protection measures.12 Leaving aside these potential risks, fluoroscopy has the disadvantage of being a 2-dimensional navigation system that allows no more than a feeling of how the catheter relates to the endocardial surface. However, in addition to dispensing completely with X-rays, 3-dimensional (3D) navigation systems can also precisely visualize the relationship between catheters and the endocardial surface. Of no less importance, these systems can also mark catheter positions on the endocardial surface to which the operator can subsequently return after exploring other points. This is something that cannot be done with fluoroscopy and is one of the main advantages of the 3D system.
The study published shows that procedures can be performed without fluoroscopy in a large number of hospitals and with different operators, some of whom have little experience with the technique. Despite this lack of experience, ablation was successful in the vast majority of patients, over 95%, with a complications rate similar to that reported for fluoroscopic procedures in Spain. Of particular note, however, was that even though more than 10 years had passed since the technique was first described by a Spanish group no less,10 and despite the possibility of use in arrhythmic substrates on the left side of the heart,13 arrhythmic procedures of this type have hardly been included in the study (2 patients with left-sided accessory pathways). Access to the left atrium often requires trans-septal puncture and there may be a risk if there is no fluoroscopic guidance. However, most of the ablation procedures on left-sided fluoroscopic substrates (accessory pathways and idiopathic ventricular tachycardias) can be performed by retroaortic artery access. The safety of this approach without fluoroscopic guidance has been questioned given the risk of inadvertently introducing the ablation catheter into the coronary artery. However, it should be highlighted that coronary arteries are also not visualized by fluoroscopy in a conventional catheterization procedure and that the navigation system probably permits greater control of this inadvertent positioning. Our group confirmed these considerations years ago in a series of 42 patients who underwent nonfluoroscopic ablation of the left accessory pathways.13 More important than the outcome itself is the fact that at the beginning of an ablation procedure without fluoroscopy, a left-sided arrhythmic substrate cannot be ruled out. This means that a fluoroscopy system needs to be available in case the substrate is indeed left-sided and nonfluoroscopic ablation of left-sided substrates is not widely practiced. Of note is that, of the 247 patients included in this study, only 2 had left accessory pathways that required the nonfluoroscopic procedure to be suspended even though this diagnosis is usually only confirmed during the catheterization procedure itself, given the difficulty of establishing the mechanism ultimately responsible for nonpre-excited tachycardias before the procedure.
For the question of whether ablation procedures require limited or no fluoroscopy, it can be argued that a few minutes of exposure to ionizing radiation are of little clinical relevance. However, certain points that refute this line of argument should be highlighted. First, the availability of fluoroscopy means that it is more likely to be used and it is harder to restrict its use in the face of the usual difficulties encountered in any procedure. Second, the human brain (or at least my brain) finds it difficult to simultaneously work with 2 geometric models of the same anatomical structure (the navigation system and the mental one resulting from movement of the catheter to different positions and radiological projections). This means that, given the long tradition of fluoroscopy use, it will be preferentially used from an early stage and the desired adoption of nonfluoroscopic navigation systems is delayed. Subsequently, in situations when this new type of navigation system would be preferred, by inertia, fluoroscopy remains engaged but not used while we concentrate on the 3D map of the navigation system. Finally, a point which may be even more important in the future is achieving total independence of the fluoroscopy system. This may appear insignificant, but it means that the procedure can continue and even get started in the event of a malfunction in the fluoroscopy system, something we have experienced over the years. Or taking a step further, we may conceive a catheterization laboratory in which a fluoroscopy system is not necessary for the simplest procedures. Fluoroscopy systems are expensive both to acquire and to maintain, as protocol requires regular checks of both technical operations and radiation exposure. The aim of eliminating fluoroscopy has been questioned with the argument that it may be necessary to have fluoroscopy available as a real-time imaging technique to visualize what is occurring in the heart (for example, visualization of an immobile cardiac silhouette in pericardial effusion). However, the simplification and broad distribution of the echocardiography systems make this type of technology less expensive and more convenient than a fluoroscopy system for this application. Perhaps the greatest limitation of not having a fluoroscopy system available is derived from possible problems with catheters, both regarding access to the heart and the great vessels from the vascular system and regarding potential complications of their use that require fluoroscopic visualization (for example, snagging or knotting). However, these complications are sufficiently infrequent that they cannot be used as the sole justification for maintaining a fluoroscopy system, especially bearing in mind that a nondedicated portable fluoroscopy system can always be used to deal with them.
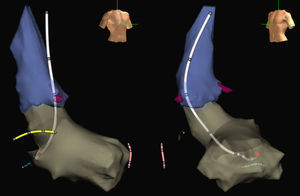
Finally, one of the problems of procedures guided by electroanatomical navigation systems without fluoroscopy is the lack of visualization of the proximal part of the catheters where there are no electrodes. Usually, placement of the ablation catheter in right heart chambers with access from the inferior vena cava does not represent a problem, given that the position of the proximal curvature of the catheter can be intuitively located. However, it is more complicated when the catheter is in left heart chambers, especially when access is retroaortic or when circular catheters are used. In this case, it is not so easy to imagine where its pole is located. This problem may lead to intermittent use of fluoroscopy. However, in recent years, designs for ablation or circular catheters have become available, including ones designed in-house.14 These enable the catheter to be visualized almost completely on the screen of the navigation system and dispense completely with fluoroscopy simply and without the need for training (Figure).
Images of the aorta (blue) and left ventricle (grey) of an electroanatomical navigation system (Ensite NavX Classic, Abbott) with oblique anterior, right projections (left) and left projections (right) obtained during a nonfluoroscopic ablation procedure of a left AV accessory pathway. The ablation catheter designed by our group (white) is shown with electrodes in its body and its tip located at the point of effective ablation (red ball) in the mitral annulus. In addition, the distal end of the catheter is shown in the Hisian region (yellow), right ventricular apex (pink), and coronary sinus (blue), also introduced without X rays. The coronary artery outlet is shown in burgundy.
J.L. Merino discloses research, consulting, and training contracts with the companies Abbott, Biosense-Webster, Boston Scientific, and Micropore.