Novel transcatheter therapies for the treatment of valvular and congenital heart disease have increased exponentially in recent years. Parallel to this trend has been the need for multi-modality imaging in the planning, guidance, and evaluation of procedure-related outcomes and complications. Echocardiography has played a pivotal role within the cardiac catheterization laboratory. Peri-procedural invasive imaging with transesophageal echocardiography (TEE), in particular three-dimensional (3D) TEE, and more recently intracardiac echocardiography (ICE), is instrumental in providing the real-time, high-resolution images of intracardiac anatomy and physiology necessary to guide a multitude of structural heart disease (SHD) interventions. With new advances in ICE, this imaging technique is an attractive alternative to TEE with potential for continued growth.
Within the last decade, echocardiography has guided an increasing number of structural heart procedures with improved success. Echocardiographic assessment of patients undergoing transcatheter therapy differs from that of patients with native or prosthetic valvular disease. The European Association of Echocardiography and American Society of Echocardiography have recognized the need for informed evaluation and have established guideline recommendations to include the newest SHD procedures: transcatheter aortic valve implantation, paravalvular regurgitation repair, and mitral valve interventions.1
As the field rapidly grows to incorporate other SHD therapies, there is an increasing demand for ultrasound imaging to be performed by the interventionalist and a reduced need for general anesthesia. In addition, there are other identifiable limitations to TEE. Visualization of more anterior structures may be limited due to a lack of far-field exposure and shadowing from surrounding structures may obscure the field of view. The TEE probe, on occasion, may partially obstruct the optimal fluoroscopic view for the interventionalist. Also, alternatives to TEE are sometimes necessary, especially in patients with absolute contraindications to esophageal intubation.
The advantage of ICE is that it has the potential to provide near-field images of cardiac structures equivalent or superior in quality to those obtained by TEE, while overcoming many of the limitations of TEE. ICE can be performed under conscious sedation and the equipment can be easily manipulated and interfaced with other interventional equipment. It provides the potential to acquire supplemental imaging not provided by TEE, further enhancing the information available to the interventionalist. ICE has also been shown to reduce procedural and fluoroscopy times and decrease overall radiation exposure to both the patient and physician.2
The first ICE transducers were described in the 1960s with one of the first descriptions of use within the cardiac catheterization laboratory in 1981. Glassman and Kronzon presented the successful use of ICE in aiding the transseptal puncture, a fundamental technique for structural heart interventions.3 In their study, an early transducer was positioned at the tip of a transseptal needle and was able to recognize when the needle made contact with the interatrial septum, suggesting that ICE was a safe and valuable adjunct to cardiac catheterization. Initial ICE catheters created cross-sectional images using a mechanically-directed, rotating transducer with operating frequencies ranging 2 to 12.5MHz. Two-dimensional images were displayed with the capability to obtain basic cardiac measurements, visualize valve structure, and measure septal defect dimensions. The 360-degree radial image provided a large field of view allowing for the visualization of many cardiac defects and their relationship to other cardiac structures. However, these catheters lacked the Doppler capabilities often required for evaluation of shunts and regurgitation.
ICE is an imaging modality in evolution. Automated rotating transducers with sector-based imaging using a 64-element phased-array (4.5 to 12.5MHz) have since been introduced. These transducers provide 90-degree sector images and have a multitude of Doppler capabilities including pulsed-wave, continuous-wave, color flow, tissue, and spectral Doppler. Image quality has been enhanced to that of TEE and penetration depths improved with the lower frequency catheters having depths of up to 21cm. In addition, these phased-array systems also provide increased maneuverability.
There are three commercially available ICE catheters. The Ultra ICE (Boston Scientific; Natick, MA, United States) is a 9F single element, rotational transducer that rotates at 1800rpm with a fixed frequency of 9MHz. It provides a 360-degree field of view, but at a radial depth restricted to 5cm, limiting the utility of this device for imaging of left-sided structures and for the transseptal puncture. Emerging applications involve crossing the septum to aid in left-sided evaluation, but given the lack of Doppler capabilities and reduced steerability they are largely utilized for electrophysiologic procedures. The AcuNav (Biosense Webster, Diamond Bar, CA, United States) is a phased-array, 8 or 10F catheter that has 64 elements used to scan in a longitudinal monoplane at frequencies ranging from 5 to 10MHz. It provides a 90-degree field of view with tissue penetration up to 16cm. The catheter is able to deflect in four planes, each at an angle of 160 degrees, offering the acquisition of multiple study planes. Lastly, the ViewFlex PLUS (St. Jude Medical, St. Paul, MN, United States) is a 9F 64 element, phased-array catheter operating at frequencies ranging from 4.5 to 8.5MHz and a penetration depth up to 21cm. It has a large curvature radius and an Agilis-designed handle that provides full maneuverability with steering angles in two planes up to an angle of 120 degrees. The two phased-array catheters provide greater frequency range, field depth, and steerabilty, and are predominately used in SHD.
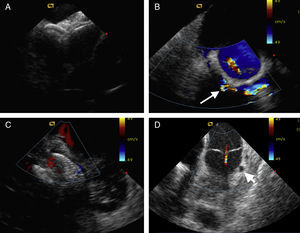
The utility of ICE has been shown for a host of SHD interventions including transseptal puncture, closure of both interatrial and ventricular septal defects, balloon valvuloplasty of the mitral, pulmonic, and aortic valves, cardiac biopsy for transplantation or of cardiac masses, septal myocardial ablation for hypertrophic obstructive cardiomyopathy, and left ventricular pseudoaneurysm closure.3, 4, 5, 6, 7, 8, 9 (Figure 1) For transseptal access, ICE provides imaging of necessary intracardiac structures such as the fossa ovalis, the posterior left atrial wall, and the aorta to confirm an optimal puncture site. With distorted or difficult anatomy, ICE guidance is increasingly important. Furthermore, many SHD procedures require precise localization of the puncture site for procedural success with examples that include a suggested 3 to 4cm transseptal access height above the mitral valve for the MitraClip delivery system (Abbott Vascular, Abbott Park, IL, United States), a posterior/superior access location for septally-located mitral paravalvular leaks for antegrade closure, and access around a prior interatrial defect closure device. Equally essential is the safe navigation through other anatomic abnormalities such a lipomatous septum, atrial septal aneurysm, and/or convex or double layer septum.
Figure 1. Examples of intracardiac echocardiography for structural heart disease interventions. A: Overlapping septal occlusion devices to treat residual shunting across an atrial septal defect. B: Mitral paravalvular regurgitation (arrow) visible around the prosthetic ring with mild transvalvular regurgitation. C: Percutaneous device closure of a muscular ventricular septal defect. D: Left anterior descending artery to pulmonary artery fistula emptying into the proximal main pulmonary artery above the pulmonic valve (arrowhead).
ICE is also being used to visualize the coronary sinus for implantation of indirect annuloplasty devices and to better delineate the size of the left atrial appendage and presence of thrombus prior to placement of a left atrial appendage occluder.10 The ability of ICE to accurately exclude left atrial appendage thrombus is questionable as visualization of left-sided structures may differ in quality dependent on the chamber location of the catheter.11 The left atrial appendage may not be well imaged from the right atrium due to variability in thickness and orientation of the interatrial septum. With increased versatility and maneuverability, the possibility of introducing the catheter within the coronary sinus, the right ventricular outflow tract or pulmonary artery can overcome the anatomic limitations of ICE by navigating closer to left-sided structures. Furthermore, the successful introduction of intrapericardial ICE for patients undergoing epicardial ablation has provided an additional route to improve image quality and guide therapy.12
Fundamental to efficacious and safe SHD interventions is the ability to apply ICE to recognize important cardiac structures, evaluate implanted device positioning, determine the presence of residual shunts and valvular hemodynamics, and prevent or recognize early complications. With ICE, potential complications can be identified and managed immediately. These complications include cardiac perforation with pericardial tamponade, thrombus formation, both native and prosthetic valvular dysfunction, and hemothorax, especially for cases where transapical access is utilized.
Nonetheless, this technology has limitations, the most significant of which includes the single-use catheter costs and the acquisition of knowledge necessary to operate the equipment. The cost of ICE catheters is high, as they are not authorized for re-sterilization. The overall costs, however, may favor using ICE, due to the lack of need for general anesthesia and anesthesiologist, reduced procedural times with shorter hospital stays and potentially reduced rates of complications.13 The lack of echocardiographer may also further decrease costs. However, the use of an echocardiographer should not be a major determinant for the use of ICE. The authors strongly believe that improved image quality and interpretation are better obtained with the presence of an echocardiographer. The integration of a multidisciplinary team improves understanding and patient care within the catheterization laboratory, especially during complex structural heart cases.
In addition, the learning curve of ICE requires the understanding of unique differences in orientation compared to standard echocardiography and the ability to manipulate the catheter within the heart to obtain the necessary working views. Several protocols have been suggested for obtaining images with ICE; however, the visualization of structures is not standardized as it is with transthoracic or TEE. The learning curve requires the operator to be familiar with its use to achieve adequate imaging and naturally builds on prior training of echocardiography with needed hands-on manipulation. For structural cases, complete interventional device visualization can be of utmost importance to guide a procedure. Due to the current bidimensional imaging with ICE, this may require careful manipulation and adjustment of the transducer position. 3D TEE, in these cases, may be required.
The large, 8 to 10F shaft sizes of ICE necessitate the use of 8 to 11F sheaths. Adequate anti-coagulation is required during the procedure to reduce thrombus formation around the catheter, with an activated clotting time maintained greater than 250. In addition, transient arrhythmias can result from direct contact of the probe with the walls of the heart. Positioning of the probe, even though rare, can interfere with simultaneous placement of treatment catheters. Repositioning may be required, but generally does not hinder the field of view necessary for therapeutic action.
Future advances in ICE include achieving higher resolution and frequency agility, 3D and real-time 3D (4D) imaging, and integration with interventional equipment while reducing catheter size. Providing 3D images with high resolution that would account for cardiac motion with both near-field views for detail and far-field views for perspective and orientation would be ideal. Multiplanar lower profile catheters and catheters integrated with computer micromachine ultrasound transducers, the next generation of ultrasound technology, are being developed.14 Computer micromachine ultrasound transducers are expected to replace the piezoelectric ceramics used in the current ultrasound technology and have inherent physical properties that can increase image resolution without increasing catheter size.
Electroanatomic mapping systems, such as CARTO (Biosense Webster), are currently able to generate 3D images by space localization with their SoundStar ICE probe (Biosense Webster).15 This probe is 10F with an ultrasound array similar to the AcuNav system and frequencies ranging from 5.5 to 10MHz. On the catheter tip is a magnetic sensor that is recognized by CARTO to triangulate the catheter tip as it is positioned inside the heart by a magnetic field. 3D anatomic reconstructions of the cardiac structure of interest can be generated and then fused with pre-procedural 3D computed tomographic angiography or magnetic resonance images. Newer systems are capable of manually reconstructing a 3D ICE image using multiple bidimensional image slices.16 These images are merged with 3D computed tomographic angiography or magnetic resonance imaging providing increased intracardiac detail and real-time, more accurate anatomic information. Lastly, achieving 4D ICE would also provide ultrasound images in real-time, rather than reconstruction. The AcuNav V (Siemens, Munich, Germany) has been presented and has shown the possibility of obtaining high-resolution volumetric images in real time. It is currently pending CE mark approval for use in Europe.
Imaging techniques for SHD are rapidly evolving. Real-time guidance with echocardiography has revolutionized transcatheter therapies by providing important high-resolution, soft tissue imaging for SHD interventions, ensuring safe and efficacious interventions. At this time, no single imaging modality is preferred, but with marked technological advances and increased use, ICE is unquestionably becoming a useful modality for the guidance of structural heart therapies. Now more than ever, it is increasingly important for the interventionalist to be trained in multimodality cardiac imaging and to operate in the context of a multidisciplinary team with imaging specialists to provide the best care for the valvular and congenital heart patient.
Conflicts of InterestNone declared.
Corresponding author: Lenox Hill Heart and Vascular Institute of New York, North Shore Long Island Jewish Health System, 130 East 77th Street, 9th Floor Black Hall Building, New York, NY 10075, United States. CRuiz@NSHS.edu