INTRODUCTION
According to the American College of Cardiology (ACC) and European Society of Cardiology (ESC) consensus document on the universal definition of myocardial infarction (MI), the cornerstone tests required for diagnosis are biomarkers such as troponins and the 12-lead ECG.1 Widespread use of troponin assays over recent years has significantly improved the diagnosis of MI and enabled the identification of patients with small infarcts, many of whom would not have been identified previously. Despite this, the diagnosis of MI can still pose problems in clinical practice. In this issue of Revista Española de Cardiología, Laraudogoitia et al2 explore the role of cardiovascular magnetic resonance (CMR) in patients presenting with features of acute MI by the consensus definition but without significant coronary artery stenosis on coronary angiography.
The Problem
A significant number of patients who have all the classical features of MI—eg chest pain, new ST-segment changes, and rise in troponins—and fulfill the universal definition, ultimately prove not to have had an MI. Indeed, several studies have demonstrated that a surprising proportion of patients with clinically suspected MI have normal coronary arteries or insignificant disease at angiography, including up to 10% of patients initially diagnosed with ST segment elevation MI (STEMI) (Table 1).3-11 Although recanalization after an occlusive coronary event is well documented,12,13 many of these patients are unlikely to have had an MI, leaving clinicians with unanswered questions regarding diagnosis and management.
It is well known that ST-segment changes and elevated troponins can occur in the setting of many disorders including myocarditis, Takotsubo cardiomyopathy, tachyarrhythmias, trauma, pulmonary embolism, sepsis, acute decompensated heart failure, drug toxicity, and acute neurological disease.1 However, the relative prevalence of these disorders is unclear as well as the best means of identification and differentiation of these conditions from MI. Thus Laraudogoitia et al2 are to be commended for shedding light on this important problem.
The Role of imaging
In these circumstances, cardiac imaging has the potential to provide important diagnostic information. This has been acknowledged in the ACC/ESC universal definition which indicates that new regional wall motion abnormalities or a loss of viable myocardium could be considered evidence of MI.1 However, it is important to realize that wall motion abnormalities may not occur unless the infarcted region exceeds 20%-50% of the myocardial wall.14,15 Similarly, scintigraphic defects in nuclear imaging may not be apparent until >10 g of myocardial tissue is infarcted.14
Thus, because a sizable threshold of damage is required, echocardiography or radionuclide imaging may miss MI, particularly when small or subendocardial. Conversely, when abnormalities in regional function or perfusion are present, they do not always indicate MI. Both may be abnormal in the setting of ischemia without infarction. Nonischemic conditions such as cardiomyopathy, inflammatory, or infiltrative diseases can also lead to regional wall motion abnormalities or loss of viable myocardium. Hence, the positive predictive value of these imaging findings are not high unless these conditions can be excluded.1
Cardiovascular Magnetic Resonance imaging
The most accurate and best validated cardiovascular magnetic resonance (CMR) technique for the diagnosis of irreversible myocardial damage is delayed enhancement-CMR (DE-CMR). This simple method involves inversion-recovery imaging approximately 10 minutes after intravenous administration of gadolinium contrast.16,17 When properly performed, normal myocardium appears black or "nulled" whereas nonviable regions appear bright or "hyperenhanced." The mechanism of hyperenhancement has not been fully elucidated, but appears to be based on the absence of viable myocytes rather than any inherent properties that are specific for acute necrosis, collagenous scar, or other forms of nonviable myocardium.17
In animal models of MI, extensive comparisons have demonstrated a nearly exact relationship between the size and shape of infarcted myocardium by DE-CMR to that of histopathology.18-22 Additionally, these studies show DE-CMR can distinguish between reversible and irreversible myocardial injury independent of wall motion, infarct age, and reperfusion status. DE-CMR has been shown to be superior to single-photon emission computed tomography (SPECT) in detecting subendocardial infarcts and infarcts in non-anterior locations.21 Furthermore, the high spatial resolution of DE-CMR enables visualization of even microinfarctions, involving as little as 1g of tissue, which may occur during otherwise successful percutaneous coronary intervention.23
Analogous to troponins, the detection of injury by DE-CMR is specific for irreversible myocardial damage but is not specific for MI. However, an advantage of DE-CMR is that the pattern of hyperenhancement, rather than simply the presence or extent, gives important information regarding the etiology of myocardial damage.24-26 For this purpose, the concept that ischemic myonecrosis proceeds as a "wavefront"27 from the subendocardium to the epicardium with increasing coronary occlusion time is crucial. Accordingly, hyperenhancement patterns that spare the subendocardium and are limited to the middle or epicardial portion of the left ventricular (LV) wall are clearly nonischemic in origin since damage in the setting of coronary artery disease almost always involves the subendocardium.24-26 Certain nonischemic disorders such as myocarditis, have characteristic hyperenhancement patterns, which may suggest a specific diagnosis, and a systematic approach to interpreting DE-CMR images in patients with cardiomyopathy has been proposed.25,28
Laraudogoitia et al2 performed DE-CMR on 80 patients that were admitted with chest pain and positive cardiac biomarkers (presumably troponins) who underwent cardiac catheterization showing "no significant lesions" within 48 hours of admission. All CMR studies were performed 4 (3) days from the time of cardiac catheterization. A diagnosis was made based on the detection of hyperenhancement in 63 patients (79%). Based on the pattern of hyperenhancement the final diagnosis was myocarditis in 51 patients (63%), and MI in 12 patients (15%). Takotsubo cardiomyopathy was diagnosed in 9 patients (11%) based on absence of hyperenhancement and initial mid-to-apical wall motion abnormalities which subsequently resolved. In 4 patients (5%) a subsequent diagnosis of pericarditis was made, presumably on clinical grounds, leaving 4 patients (5%) with no specific diagnosis. It is important to point out that the authors' use of the term "no significant lesions in the coronary arteries" is unclear. This is of interest since it may refer to stenosis <70% or to completely normal vessels without luminal irregularities.
These findings are consistent with the recent work of Assomull et al who evaluated the role of CMR in 60 patients presenting with chest pain, elevated troponins, and unobstructed coronary arteries (<50% stenosis).29 DE-CMR provided a new diagnosis in 65% of patients. The commonest underlying cause was myocarditis (50%), followed by MI (12%). However, in contrast to the present study only 1 patient (1.7%) had the diagnosis of Takotsubo cardiomyopathy made.
Similarly, in a recent registry of 1335 STEMI patients undergoing coronary angiography, Larson et al reported that 14% had no culprit artery and 9.5% did not have significant CAD (<50% stenosis).3 The majority of patients with no clear culprit artery and positive cardiac biomarkers underwent CMR, which established that the most common diagnoses in this subgroup were myocarditis (31%), Takotsubo cardiomyopathy (31%), and STEMI without an angiographic lesion (29%).
Takosubo Cardiomyopathy
The diagnosis of Takosubo cardiomyopathy is dependent on the presence of typical apical wall motion abnormalities in the absence of significant obstructive CAD which resolves over a period of several weeks or months. The study of Laraudogoitia et al2 suggests the potential role of CMR in helping to distinguish these patients from those with myocarditis or MI by demonstrating the absence of hyperenhancement. Eitel et al recently examined the role of CMR in 59 patients presenting with typical clinical features of Takosubo cardiomyopathy (acute coronary syndrome patients without significant obstructive CAD and typical apical wall motion abnormalities).30 Interestingly, DE-CMR demonstrated an alternative diagnosis in 21 patients (35.6%). The DE-CMR pattern showed MI in 13 patients (22%) and myocarditis in 8 patients (13.6%). Therefore only 38 patients (68%) were given the diagnosis of Takosubo cardiomyopathy on the basis of CMR. Follow-up CMR was performed in 32 of these patients (84%) and showed completely normalized systolic function.
Acute Myocardial infarction
The present study is consistent with prior observations regarding the presence of acute MI in a significant number of patients, despite unobstructed arteries. The diagnosis is based on subendocardial or transmural hyperenhancement patterns in coronary distributions with the presence of microvascular obstruction in some cases. Laraudogoitia et al2 did not explore the possible causes of acute MI in these patients which may include coronary embolism, spasm, or recanalization. In this regard, DE-CMR is highly sensitive for the detection of intra-cardiac thrombus when performed correctly,31 and in our own practice we have found previously unknown intra-cardiac thrombi in a number of these patients. In the presence of unobstructed coronary arteries, the finding of intra-cardiac thrombus would strongly suggest coronary embolism as the underlying cause. We have also observed cases where the presence of acute MI by DE-CMR prompted re-reviewing of the original coronary angiogram revealing a (usually small) occluded artery which had been originally overlooked.
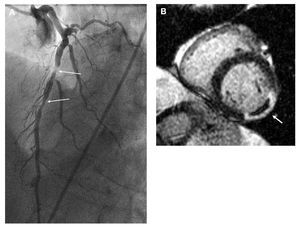
In many patients with acute MI or acute coronary syndrome the culprit artery cannot be identified (Table),3-11 because of the absence of CAD, absence of typical angiographic characteristics, or because multivessel CAD is present and more than one artery/culprit could be responsible. In these circumstances, by visualizing the MI location, DE-CMR may be helpful in identifying the infarct related artery. The primary utility of this would be in patients with multivessel disease (Figure) since the practical implications of identifying the infarct related artery in the absence of significant stenosis are currently unclear.
Figure. Identification of the infarct related artery using cardiovascular magnetic resonance (CMR). A 69 year old man presented with a several week history of exertional chest discomfort while running. On the morning of admission he experienced chest pain whilst sitting in church. His ECG showed no significant signs of ischemia but he had a positive troponin T. (A) Cardiac catheterization showed a 90% mid left anterior descending (LAD) lesion and a sequential 70% mid-distal LAD lesion (arrows) with a proximally occluded circumflex artery. There was only non-obstructive disease in the right coronary artery. Two drug eluting stents were placed in the LAD with the assumption that the circumflex was chronically occluded and the LAD was the acute infarct related artery. (B) Subsequent CMR demonstrated trans-mural hyperenhancement with an area of microvascular obstruction in the infero-lateral wall (arrow) consistent with an acute infarct in the territory of the circumflex artery.
T2-Weighted imaging
T2-weighted imaging has shown promise in assessing acute, edematous, inflammatory processes, such as acute MI or myocarditis and may prove useful in distinguishing chronic myocardial lesions from those of recent onset.32 However, it is interesting to note that T2-weighted imaging did not appear to significantly improve diagnostic performance over and above that provided by DECMR and cine imaging in the present study. Only 37 of 51 patients with evidence of myocarditis and 3 of 13 patients with evidence of MI on DE-CMR showed increased T2 signal intensity. Similarly, just 3 of 9 patients with Takosubo cardiomyopathy and apical wall motion abnormalities showed increased T2 signal intensity. Laraudogoitia et al2, speculate that the late imaging of some of the patients may have been responsible for the low yield of T2-weighted imaging in their study. However, all CMR studies were performed within 4 (3) days from the time of cardiac catheterization which is well within the acute period of tissue edema. This relatively low sensitivity of T2-weighted imaging is also consistent with the findings of both Assomull and Eitel, and likely relates to the limitations of current black-blood T2-weighted imaging sequences.29,30,33
CONCLUSIONS
The study of Laraudogoitia et al2 adds to the growing body of work demonstrating the utility of CMR in patients presenting with signs and symptoms consistent with acute MI but absence of obstructive CAD on coronary angiography. This clinical conundrum occurs not infrequently and adds to the increasing number of indications for CMR in daily clinical practice. Future work should focus on the prognostic significance of the CMR findings in this patient group. In addition, rapidly evolving technical advances in several areas including newer T2-weighted and delayed enhancement sequences as well as more rapid acquisition times may improve the diagnostic yield and usefulness of CMR in these settings.
See original on pageS 976-83
Conflicts of interests disclosures: Dr. Raymond J. Kim is an inventor of a US patent on delayed enhancement cardiovascular magnetic resonance that is owned by Northwestern University. Dr. Raymond J. Kim is a co-founder of HeartIT, LLC.
Correspondence: R. J. Kim, MD,
Duke Cardiovascular MRI Center,
DUMC 3934, Durham, NC 27710, USA E-mail: raymond.kim@duke.edu