Keywords
High-density lipoprotein (HDL) is today regarded as one of the most important protective factors against arteriosclerosis. Traditionally, HDL's protective function has been attributed to its active participation in the reverse transport of cholesterol. Since 1975, numerous cohort studies and clinical trials have confirmed the association between a low HDL-cholesterol concentration and an increased risk of coronary heart disease.15 In experimental animals, an inverse correlation between HDL concentration and the development of arteriosclerosis has been demonstrated. However, it has been observed that arteriosclerotic lesions tend to regress in vivo as the concentration of HDL or its apolipoproteins increases.68 On the other hand, certain genetically inherited diseases that are characterized by an abnormally low HDL level, such as Tangier disease, fish-eye disease and diseases linked to apo A1 gene mutations, are frequently associated with arteriosclerosis and premature ischemic heart disease.912 Recently, it has been shown that increasing the HDL-cholesterol concentration is artificially inhibited by cholesterol ester transfer protein. However, the effect an increased concentration has on the risk of coronary disease remains unknown.13
The "French paradox" in cardiovascular disease refers to the coexistence of a diet rich in saturated fats with low mortality from coronary heart disease. In Spain, despite the fact that coronary heart disease affects more than 68 000 people annually and costs the lives of more than 40% of those individuals, it has also been confirmed that a low incidence of acute myocardial infarction (AMI) coexists with a high prevalence of classic cardiovascular risk factors.14,15 For this reason, it makes sense to study the variables that could explain this remarkable situation, including, importantly, HDL and it properties. The significant antioxidant and anti-inflammatory effects of HDL, which have been discovered in the last decade, could substantially modify our understanding of the mechanisms that prevent arteriosclerosis.
In the Spanish population, HDL concentrations are similar to or slightly higher than those normally found in individuals from countries with a higher incidence of coronary heart disease, such as the United States, Australia, and the countries of northern Europe.16 Consequently, the absolute HDL concentration does not appear to be the determining factor in explaining the observed geographical differences in disease incidence.16 It could be that specific qualitative characteristics of the HDL particle increase HDL's antioxidant capacity and, thereby, enable it to provide greater protection against cardiac ischemia.16 Paraoxonase-1 (PON1), the enzyme responsible for HDL's antioxidant function, is closely bound to the HDL particle. PON1's enzymatic activity is highly regulated by environmental factors such as diet and physical activity, by certain drugs, and by genetic factors, especially certain genetic polymorphisms in the paraoxonase-1 gene, PON1.1719 The antioxidant capacity of PON1 and the variability observed in its activity, which can be evaluated using the paraoxon substrate in a variety of contexts, ensure that the results of investigations into PON1 will be of great interest in the field of arteriosclerotic disease.
PROPERTIES AND CHARACTERISTICS OF PARAOXONASE-1
PON1 is synthesized in the mammalian liver and circulates in blood bound to HDL apolipoprotein (apo) A-1 and apo J. PON1 expression is inhibited by proatherogenic stimuli.2023 There are 2 other proteins in the same family that probably also have an antioxidant function: PON2 and PON3. PON2 is ubiquitously expressed within cells, whereas PON3 exhibits a basal constitutive antioxidant activity and is essentially bound to HDL.22,2426 In this review, we will focus on PON1, whose relationship with numerous pathological conditions and with environmental and genetic factors has been the most extensively studied of the 3 proteins.
PON1 has a number of enzymatic activities (Table). We will concentrate on its antioxidant activity and its paraoxonase activity, that is, the activity that is quantified using the pesticide paraoxon as a substrate.
Paraoxonase Activity (Paraoxon Substrate)
PON1 was first identified in the field of toxicology because it was able to hydrolyze synthetic organophosphates such as pesticides (e.g., paraoxon, and oxon metabolites of chlorpyrifos and diazinon) and nerve gases (e.g., sarin and soman).27 Paraoxon is a toxic metabolite produced in the liver from parathion, which is a relatively innocuous compound.28 In mammals, when paraoxon enters the bloodstream, it is hydrolyzed and inactivated by HDL PON1. This does not occur in birds, fish and insects, which generally lack PON1 and are easily poisoned by these compounds.29
It is important to note that paraoxonase activity is reduced in patients with conditions associated with arteriosclerosis, such as AMI, diabetes mellitus and familial hypercholesterolemia (FH).3033 It has been reported that PON1's level of interaction with the paraoxon substrate is highly correlated with the protein's concentration.34 However, despite strong binding of the enzyme to the HDL particle, there does not always appear to be a correlation between HDL concentration and paraoxonase activity.35
Other Enzymatic Activities of Paraoxonase-1: Arylesterase (Phenylacetate Substrate) and Lactonase (Lactone Substrate) Activities
PON1 is able to hydrolyze arylesters such as phenylacetate by means of its arylesterase activity, which also appears to be related to the concentration of the protein.34,36,37 It also has the capacity to hydrolyze more than 30 different lactone molecules, including endogenous molecules, such as homocysteine thiolactone and glucocorticoid gamma-lactones, and exogenous molecules, such as statins.38,39
Antioxidant Activity
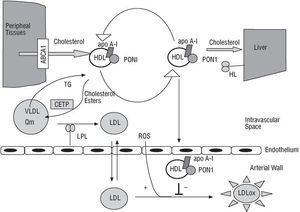
PON1 endows HDL with its antioxidant properties and is probably responsible for the principal mechanism inhibiting the oxidation of both low-density lipoproteins (LDLs) and HDL itself, a process that is directly involved in the initial phases of arteriosclerosis, as illustrated in Figure 1.4043 In vitro, PON1 neutralizes hydrogen peroxide and peroxidized lipids that are either free or present in atherosclerotic lesions or in minimally oxidized LDL.42,4446 However, although there is still no general agreement on the subject, PON1 may be able to activate platelet activating factor acetylhydrolase, an enzyme that hydrolyzes this well-known proinflammatory factor. This process could give PON1 its anti-inflammatory properties.47,48
Fig. 1. The antioxidant role of paraoxonase-1 in arteriosclerotic plaque formation. ABCA1 indicates ATP-binding cassette A1; apo A-I, apolipoprotein A-I; CETP, cholesterol ester transfer protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDLox, oxidized LDL; HL, hepatic lipase; LPL, lipoprotein lipase; PON1, paraoxonase-1; Cm, chylomicron; ROS, reactive oxygen species; TG, triglycerides; VLDL, very-low-density lipoprotein.
Experimental evidence for the antioxidant function of PON1 comes from in vivo studies carried out in PON1/apo E double knock-out mice. These studies have demonstrated that PON1 is effective in inhibiting the oxidation of both LDL and intermediate-density lipoproteins, substances with proven atherogenic effects.49 PON1-knock-out mice fed an atherogenic diet rich in fat and cholesterol developed more arteriosclerosis than wild-type mice. In addition, their HDL was incapable of counteracting LDL oxidation, being both LDL and HDL itself more easily oxidized.18 Animals lacking the PON1 gene are in a state of increased oxidative stress.50,51 In contrast, mice that overexpress PON1 demonstrate less lipid hydroperoxide production.52 Moreover, when human PON1 is injected into apo E-knock-out mice that exhibit accelerated arteriosclerosis development, the lipid peroxide content of peritoneal macrophages decreases significantly.53
Factors Regulating the Enzymatic Activity of Paraoxonase-1
Lipid peroxides inhibit the paraoxonase, arylesterase and antioxidant activities of PON1, probably via interactions with a sulfur group on the enzyme.54,55 One important consequence of this phenomenon is that, if HDL is oxidized, there will be an accompanying reduction in paraoxonase activity and, therefore, also a reduction in the enzyme's protective activity against LDL oxidation.56
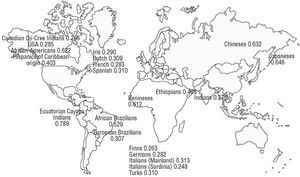
In addition to being influenced by lipid peroxides, the variations observed in PON1's enzymatic activity also depend on specific amino acids in the protein (such as a cysteine at position 283), the plasma concentration of the calcium ion Ca2+, and the presence of genetic variants in gene-coding regions, such as PON1-192 and PON1-55, and in the gene promoter, such as PON1-(107).38,54 Figure 2 illustrates the frequency of occurrence of the R allele of the PON1-192 polymorphism in different parts of the world.
Fig. 2. The frequency of occurrence of the R allele of the PON1-192 polymorphism in different parts of the world. Adapted from Scacchi et al159 with permission (© 2003 Wayne State University Press, Detroit, Michigan, USA). Data on the Spanish population are taken from Senti et al.134
PARAOXONASE-1 AND DISEASES RELATED TO ARTERIOSCLEROSIS
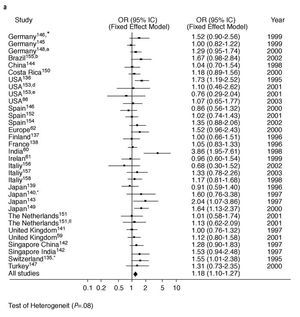
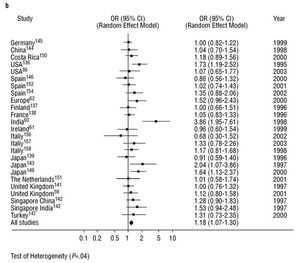
Diseases associated with arteriosclerosis and with various environmental factors are, to a large extent, related to by PON1 activity. However, PON1 is known to be an inducible enzyme as various stimuli can modify its expression.22 To date, the published results of studies into the relationship between specific genetic polymorphisms in the PON1 gene and coronary heartdisease have been clearly inconsistent. This is the case with the PON1-192 polymorphism. We carried out a meta-analysis on this polymorphisms as part of this review. See Figure 3. The principal finding was that the presence of the R allele of the PON1-192 polymorphism was weakly but significantly associated with the presence of coronary heart disease when the results of all the studies that dealt with the subject were combined. This finding was not affected by whether (Figure 3a) or not (Figure 3b) the studies looked at certain subgroups of patients, such as those with diabetes or FH, or those who belonged to a specific age group. It would seem logical, therefore, that PON1 genotype, as well as PON1 activity and concentration, must be taken into account in attempting to predict the risk of coronary heart disease.19,57,58 However, to date, few studies have looked at PON1 polymorphisms in combination with paraoxonase or arylesterase activities.5962 Nevertheless, there are some indications that the interindividual variability in PON1 activity found in healthy subjects is principally due to factors that are independent of genotype.63
Fig. 3a. Meta-analysis of the relationship between the frequency of occurrence of the R allele of the PON1-192 polymorphism and the presence of coronary heart disease in 33 studies. *Diabetics; aage <62 years; bage <45 years; dprospective placebo-controlled study; eprospective study of fluvastatin treatment; ¼familial hypercholesterolemia; OR indicates odds ratio; CI, confidence interval
Fig. 3b. Meta-analysis of the relationship between the frequency of occurrence of the R allele of the PON1-192 polymorphism and the presence of coronary haert disease in 25 studies that did not include diabetics, that did not have an age limit, that did not involve prospective assessment of interventions, and that did not compare control subjects with patients with familial hypercholesterolemia. OR indicates odds ratio; CI, confidence interval
Coronary Heart Disease, Inflammation and Paraoxonase-1
It is well established that arteriosclerosis and its clinical manifestations are often associated with a reduced concentration of HDL, the type of lipoprotein on which PON1 is located. In fact, we know that a low HDL concentration is usually accompanied by a reduction in PON1 activity or concentration, such as occurs in Tangier disease and fish-eye disease.64,65
Cellular factors associated with inflammation have been found in arteriosclerotic lesions. Some studies have shown that the incidence of arteriosclerosis is related to the presence of certain infectious agents, such as Chlamydia pneumoniae and cytomegalovirus, and to the presence of conditions such as chronic bronchitis.6669 Moreover, certain molecules implicated in acute-phase inflammatory responses, such as interleukin-1 (IL-1), IL-6, endotoxins, and oxidized phospholipids, reduce both PON1 expression and its paraoxonase activity.70,71 Similarly, it has been reported that proinflammatory cytokines, such as IL-1β, tumor necrosis factor-alpha (TNF-α), and IL-6, have a coordinated effect on the regulation of PON1 transcription in hepatocytes.72 The formation of immune complexes directed against oxidized apo A-I that has been observed in mice fed on a short-term atherogenic diet could be the mechanism by which oxidized HDL is eliminated. This process could lead to a reduction in paraoxonase activity without affecting messenger RNA (mRNA) levels.73
Reduced paraoxonase activity and the concomitant loss of HDL's antioxidant properties have been observed during the acute-phase response in patients who have undergone cardiac surgery.74 In addition, reduced paraoxonase activity has also been observed in subjects with antiphospholipid autoantibodies, in individuals infected by the influenza virus, and in patients with rheumatoid arthritis.7577 In these individuals, mortality from coronary heart disease appears to be increased.
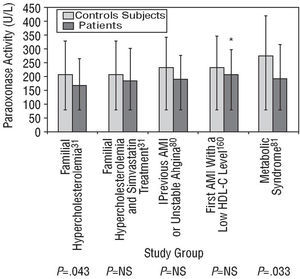
Paraoxonase activity is diminished after an AMI and remains reduced from 2 hours to approximately 40 days after the event.30,32,78 Moreover, activity may also be diminished for a short time before the onset of symptoms. Serum arylesterase activity, which declines during heart failure, increases again after appropriate treatment is given.79 In addition, diseases that are closely linked to arteriosclerosis, such as FH, the metabolic syndrome and AMI, are also associated with reduced paraoxonase activity, as was observed in some of the studies illustrated in Figure 4.31,33,80,81 Nevertheless, in the absence of an analytical test based on lipid peroxide hydrolysis and of accurate knowledge of PON1's physiological substrate, it would make sense to determine paraoxonase and diazoxonase activity or the PON1 concentration in all epidemiological studies looking at the relationship between PON1 and coronary disease.34,59
Fig. 4. Paraoxonase activity (mean ± standard deviation) in untreated patients with familial hypercholesterolemia, in patients with familial hypercholesterolemia receiving simvastatin, in stable patients with a history of acute myocardial infarction (AMI) or unstable angina, in patients who had had a first AMI 6 months previously and who had a low high-density lipoprotein cholesterol (HDL-C) level, and in patients with the metabolic syndrome, each compared with healthy control subjects. P indicates significance of the statistical difference between patients and control subjects; NS, not significant; *median and interquartile range.
Individuals with a paraoxonase activity level in the highest quintile have less risk of coronary heart disease than those whose activity level lies in the lowest quintile. In addition, the difference was found to be particularly striking in patients taking part in studies on the secondary prevention of coronary heart disease.82 The paraoxonase concentration and paraoxonase activity are reduced by as much as 50% in patients with coronary heart disease, irrespective of whether they have the PON1-192 or PON1-55 genotype.59,83 Moreover, some of these patients have normal HDL levels. It has been suggested that their type of HDL is insufficiently protective against monocyte migration to atherosclerotic plaque.84 In addition, it has been observed that the PON1 concentration tends to be lower in populations in which there is a high prevalence of coronary heart disease.62 In this context, it is worthwhile noting that the PON1 concentration predicts the occurrence of coronary heart disease in a similar way to other parameters, such as the ratio of the apo J concentration to paraoxonase activity or the ratio of the total cholesterol concentration to the HDL cholesterol concentration.59,84
Certain pecularieties have has also been reported in patients who present with chronic complaints that are frequently associated with coronary heart disease exhibit certain peculiarities. For example, renal transplant patients, with or without coronary heart disease, have reduced levels of paraoxonase and arylesterase activity.61 In contrast, diabetic patients with coronary heart disease present with higher PON1 activity levels than those without coronary heart disease.37
Some authors regard paraoxonase activity as being a better predictor of coronary heart disease risk than the PON1-(-107), PON1-55, or PON1-192 genotypes alone. Nevertheless, it is important to remember that enzyme activity levels overlap considerably between patients with different genotypes of the same polymorphism.57,59,85,86
Familial Hypercholesterolemia and Paraoxonase-1
The prevalence of the heterozygotic form of FH in the general population is 0.2%. The disease is characterized by a reduced number of LDL receptors, which is due to mutations in the receptor gene. The majority of patients with FH present with type-IIa hyperlipidemia with a smaller proportion presenting with phenotype-IIb hyperlipidemia, where the different forms of hyperlipidemia are defined according to the World Health Organization's adaptation of Fredrickson's hyperlipidemia classification.87 Affected patients often have early arteriosclerosis.88,89 It has been observed that paraoxonase activity in patients with FH is 50-80% of that found in unaffected individuals despite there is no difference in HDL concentration.31,90 A similar situation occurs when the hypercholesterolemic phenotype is reproduced in animals fed on a saturated fat-rich diet. These animal models demonstrate significant reductions in paraoxonase activity in the absence of any appreciable change in HDL level.91
Statin treatment has been observed to increase paraoxonase activity significantly in patients with FH.31,92,93 Factors associated with transcription may by involved in this phenomenon since it is known that simvastatin can increase the transcriptional activity of the PON1 gene promoter by 2.5 times through a mechanism that depends on mevalonate, a metabolic product of 3-hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase.94
Statins are hypolipidemic compounds that inhibit HMG-CoA reductase, a key enzyme in the intracellular biosynthesis of cholesterol. In general, when used for either primary or secondary disease prevention, statins bring about a reduction in total and LDL cholesterol levels, and alter the HDL concentration to a variable extent.9598 In addition to modifying the lipid profile, simvastatin, like other statins, can have pleiotropic effects that are not directly related to lipids. These can either prevent arteriosclerosis or bring about its regression by means of a variety of mechanisms.99 In particular, both in vitro and ex vivo experiments indicate that simvastatin itself can act as an antioxidant. Adding simvastatin to a medium containing LDL and HDL reduces the rate of formation of conjugated dienes, products of fatty acid oxidation. Similarly, ex vivo experiments in patients treated with simvastatin show that there is less oxidation of their LDL and HDL.95 Moreover, PON1 can hydrolyze statins, which contain lactone rings.38 Some derivatives of atorvastatin have been observed to inhibit LDL and HDL oxidation in vitro and to maintain paraoxonase activity.100 This latter effect has not been observed with fluvastatin, whose administration is associated with a reduction in PON1's paraoxonase, arylesterase and lactonase activities in rats. However, fluvastatin does reduce oxidative stress in these animals.101
The fibrates form another group of widely used hypolipidemic medications. Their principal pharmacological actions are to reduce the triglyceride concentration and to increase the HDL cholesterol concentration. Although some serious side effects have been reported, combined statin and fibrate administration may be indicated in patients with hypercholesterolemia and hypertriglyceridemia.89 Numerous clinical trials have confirmed that fibrates can slow arteriosclerosis progression and can reduce cardiovascular morbidity and mortality. These benefits are due to the compounds' hypolipidemic activity and possibly also to their pleiotropic effects, which are anti-inflammatory and antioxidant in nature and can improve endothelial function.102
There is no general agreement about how fibrates influence paraoxonase activity. Although, initially, gemfibrozil was reported to increase paraoxonase activity, this observation has not been duplicated in later studies on gemfibrozil, bezafibrate, ciprofibrate or fenofibrate.103106
Nevertheless, some reports indicate that gemfibrozil helps preserve paraoxonase activity and inhibits LDL and HDL oxidation in vitro.100,107
Diabetes Mellitus and Paraoxonase-1
Type-I diabetes mellitus is associated with an elevated level of oxidative stress, increased susceptibility to coronary heart disease, and reductions in PON1 concentration and paraoxonase activity.108 It has been reported that 67% of type-I diabetics have a low level of paraoxonase activity, irrespective of HDL cholesterol concentration, compared with 50% of the healthy population.90
Type-II diabetes mellitus is characterized by a raised blood glucose level, hypertriglyceridemia, increased oxidative metabolism, reduced HDL cholesterol concentration, high prevalence of obesity, and accelerated arteriosclerosis.109111 Type-II diabetes is also associated with the occurrence of cardiovascular events, which are probably linked to low HDL cholesterol concentrations rather than to elevated LDL cholesterol concentrations.112 Moreover, type-II diabetics present with a lower paraoxonase activity level and a lower ratio of paraoxonase activity to HDL cholesterol level than those found in health control subjects.113115 One striking observation in these patients is that there is no correlation between paraoxonase activity and HDL cholesterol concentration.115 In addition, diabetic patients who present with complications such as coronary heart disease, retinopathy or neuropathy37,114,116 have lower paraoxonase activity levels than diabetics without these complications.90,113,117 In our laboratory, we have recently observed that paraoxonase activity decreases as the number of metabolic alterations linked to the metabolic syndrome rises and that this decrease is accompanied by increasingly high concentrations of lipid peroxides.81 Both individuals with impaired glucose tolerance and recently diagnosed diabetics have normal enzymatic activity levels even though they exhibit an abnormally high level of LDL oxidation.118 Apparently, paraoxonase activity declines as diabetes progresses and reaches a particularly low level in advanced stages of the disease. In patients with obesity associated with an elevated leptin concentration, reduced PON1 paraoxonase, arylesterase and lactonase activities and increased oxidative stress have been observed. These phenomena could explain, at least in part, the relationship between obesity and arteriosclerosis in hyperleptinemics.119 It has also been observed that paraoxonase activity and HDL concentrations are lower in diabetic patients undergoing hemodialysis than in non-diabetic patients undergoing hemodialysis.120
In vitro, an elevated glucose concentration reduces HDL's antioxidant capacity, partly because both paraoxonase activity and the ratio of paraoxonase activity to HDL cholesterol level decrease while the concentration of oxidation markers simultaneously increases.117 In rats with streptozocin-induced diabetes, serum paraoxonase activity declines progressively with time.121
Neurological Disease and Paraoxonase-1
The majority of organophosphate compounds are neurotoxic. Chronic exposure can induce neuropathies and has neuropsychiatric effects.122 It has been suggested that PON1's ability to detoxify both organophosphates and lipid peroxides could make the enzyme a key element in determining susceptibility to neurological disease, such as Alzheimer disease and Parkinson disease.123
One notable observation is that farmers who disinfect sheep with organophosphates that have diazoxon as a metabolite can become ill if PON1's ability to hydrolyze diazoxon is reduced.124 Low PON1 activity levels have been observed in Persian Gulf War veterans, some of whom presented with neurotoxic effects.125,126
Kidney Failure and Paraoxonase-1
Chronic kidney failure is generally associated with dyslipidemia, an elevated risk of coronary heart disease, and an elevated level of oxidative stress, which is probably due to the inadequate antioxidant enzymatic activities of superoxide dismutase, catalase and PON1.127129 Although paraoxonase activity, aryles terase activity and the HDL level are all reduced in patients who are undergoing hemodialysis or who have uremia, this not the case for the ratio of paraoxonase activity to HDL cholesterol level or for the ratio of paraoxonase activity to apo A-I level in diabetics with nephropathy.113,129132 Kidney transplantation appears to re-establish normal paraoxonase activity and may also increase arylesterase activity.61,133
CONCLUSIONS
The ability of HDL to protect against arteriosclerosis and coronary heart disease has traditionally been attributed to its well-known participation in the reverse transport of cholesterol. Within the last few years, another mechanism has been discovered: HDL's function as a LDL antioxidant. This additional property of HDL could be vital since its effect is to prevent or delay the formation of atheromatous plaque. Consequently, our understanding of the mechanisms by which HDL exerts a protective effect has been substantially refined. It is now believed that it is not only the quantity of HDL that is important but also the qualitative nature of its antioxidant capacity. The presence in HDL particles of the antioxidant enzyme PON1, which determines HDL's ability to restrain the process of LDL oxidation in subendothelial tissue, strengthens this belief. If, as it appears, the antioxidant capacity of HDL depends almost exclusively on PON1, it is logical to search for factors that can bring about an increase in PON1 activity. This discovery clearly has important clinical implications and may contribute to explaining part of the southern European paradox, which is illustrated, for example, by the fact that both the mortality and the incidence of myocardial infarction in Spain are lower than would be expected from the observed prevalence of known risk factors.
Full English text available at: www.revespcardiol.org
ABBREVIATIONS
AMI: acute myocardial infarction.
apo: apolipoprotein.
FH: familial hypercholesterolemia.
HDL: high-density lipoprotein.
HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A.
IL: interleukin.
LDL: low-density lipoprotein.
mRNA: messenger ribonucleic acid.
PON: paraoxonase.
TNF: tumor necrosis factor.
This review was partly funded by grants FIS 99/0013/01, FEDER 1FD97/0626, and FIS G03/45 (Red Temática de Grupos HERACLES) awarded by the Instituto de Salud Carlos III, Spain, and by grant FIS C03/01 (Red Temática de Centros RECAVA) awarded by the Fondo de Investigación Sanitaria, Spain.
Correspondence: Dr. J. Marrugat.
Unitat de Lípids i Epidemiologia Cardiovascular.
Institut Municipal d'Investigació Mèdica (IMIM).
Dr. Aiguader, 80. 08003 Barcelona. España.
E-mail: jmarrugat@imim.es