Keywords
INTRODUCTION
Aortic dissection is an unusual complication of percutaneous transluminal coronary angioplasty (PTCA).1-8 Most cases described in the literature occurred after interventions addressing the right coronary artery1,3-10 and, very rarely, after left coronary artery catheterization.1,3,4,6 This complication is potentially serious and can lead to acute myocardial infarction or sudden cardiac death.
Using dissection and histological sections, we studied the course and morphological and structural characteristics of the coronary sinuses and the proximal part of the left and right coronary arteries in post-mortem specimens with and without structural ischemic heart disease, with the aim of studying the mechanisms and factors that can make the left coronary artery less prone to retrograde dissection than the right during PTCA.
METHODS
We studied 16 post-mortem hearts that had been previously fixed by immersing them in 10% buffered neutral formalin, while avoiding doing this under pressure via the coronary arteries as this could have distended them and led to the samples becoming distorted. The causes of death were associated with: road traffic accident (n=6), cirrhosis of the liver (n=2), suicide (n=3), cerebral hemorrhage (n=3), and pulmonary thromboembolism (n=2). In total, 8 of the 16 specimens presented structural ischemic heart disease with stenosis of the right and left coronary arteries due to atherosclerosis. There were 10 male and 6 female patients, 30-78 years old (mean ± standard deviation, 55±9 years). The weight of the hearts ranged between 332 and 450 g (380±22 g); the hearts were dissected to locate the origin of the coronary arteries (16 samples in the right coronary artery and 14 in the left) and their proximal course. Using a goniometer, we measured, macroscopically or in the histological sections, the angle between the ostium of origin and the proximal part of the right and left coronary artery and the aortic wall. Following this, the histological sections were made via sectioning the ascending aorta horizontally 1.5 cm above the sinotubular junction. Afterwards, the aorta was sectioned again lengthwise through the non-coronary sinus to allow easier examination of the coronary orifices. The sinuses of Valsalva, including the proximal part of the coronary arteries, were resected and processed to create histological sections. Two blocks were made from each heart, approximately 7 mm thick, which were dehydrated in graded alcohol, embedded in paraffin, and sectioned consecutively at 10 µm in the frontal plane. The sections were stained at 60 µm intervals using Masson trichrome and picrosirius red F3BA (Gurr, United Kingdom) protocols at 1% dilution. Using a polarized light microscope, the collagen fibers stained with picrosirius red present birefringence, indicating the presence of submicroscopic units oriented along the fiber axis. These subunits are made up of type I and II collagen. Type I is strongly birefringent, with colors ranging from yellow to red; on the other hand, type III collagen is less refringent and appears green.
The structures of the histological sections were measured using image analysis software (SigmaScanPro 5.0, Jandel Scientific, San Rafael, CA, USA). To aid in visualizing the coronary ostia architecture and the ascending aortic wall, we used a scanning electron microscope (Jeol JSM 5600) to examine the 25-30 µm histological sections that had previously been deparaffinized in xylol for 30 min, then afterwards dried at room temperature for 1 h, and covered with gold (BAL-ECT SDC 005 Sputter coater) for 4 min.
Statistical Analysis
The results are expressed as mean ± standard deviation (SD). Statistical analysis was done using the Student t test for independent samples in the case of quantitative variables. P values <.05 were considered statistically significant.
RESULTS
Morphology of the Aortocoronary Junction and Its Proximal Tract
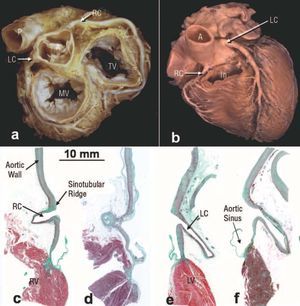
All the hearts studied had 3 aortic leaflets and the coronary arteries originated in the corresponding sinuses of Valsalva (Figure 1). Aortic sinus diameters were 3.7±0.3 cm (range, 2.6-4.2 cm). The right coronary artery ostium (12 hearts, 75%) and the left (12 hearts, 85%) were located below the sinotubular junction. There were significant differences between the diameters of the left and right coronary artery ostia. The diameter of the left coronary artery ostium in 10 specimens (71%) was greater (4.5±0.4 mm) than the right coronary artery ostium (3.7±0.5 mm) (P<.001). Using dissection and optical microscopy techniques, all the specimens showed that the ostium and the first 2-3 mm of the coronary arteries were located within the wall of the ascending aorta or the aortic sinus. From here, the initial extraaortic part (approximately 2 cm) of the left coronary artery descends parallel to the ascending aorta and forms, with the aortic sinus wall, an angle of 35.5o±11.5o (range, 20o-55o), to course between the pulmonary trunk and the left atrial appendage (Figure 1). In contrast, the initial part of the right coronary artery originates almost perpendicular to the aortic sinus wall forming an angle of 71.5o±8.5o (range, 60o-88o), and passes in front of and slightly to the right of the right atrium, lying between this and the trunk of the pulmonary artery (Figure 1). As it approaches the atrioventricular groove, the right coronary artery descends almost vertically.
Figure 1. Image of the base (a) and anterior wall (b) of the heart in the anatomical position showing the aorta (A) and the course of the coronary arteries through the atrioventricular groove. Note that the proximal part of the left coronary artery (LCA) has a route different to that of the right coronary artery (RCA). In figure b the infundibulum (In) or the outflow tract of the right ventricle has been sectioned. c-f: frontal sections through the ostium and initial part of the coronary arteries: right coronary artery control (c), atherosclerotic right coronary artery (d), left coronary artery control (e), and atherosclerotic left coronary artery (f) stained with Masson trichrome. Note that the left coronary artery forms a more acute angle with the aortic sinus than the right and both ostia are located below the sinotubular junction. Bar, 10 mm. RCA indicates right coronary artery; LCA, left coronary artery; PA, pulmonary artery; RV, right ventricle; LV, left ventricle; MV, mitral valve; TV, tricuspid valve.
Histological and Structural Study of the Coronary Artery Ostia and the Aortic Wall
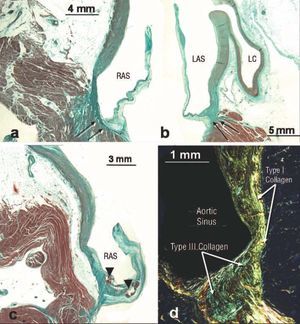
Using conventional, polarized light and scanning electron microscopy, histological examination showed that the walls of the sinuses of Valsalva are basically made up of type I collagen in their lower part proximal to where the aortic leaflets attach, where muscle fibers insert into the left ventricle (Figure 2); however, the number of type I collagen fibers decrease as the elastic fibers in the ascending part of the aortic sinuses increase (Figure 2). The aortic wall thickness was 1.8±0.3 mm (range, 1.1-2.4 mm) in the medial portion of each sinus, both in the normal hearts and in those presenting structural ischemic cardiopathy. However, the sinuses where structural ischemic cardiopathy was found presented non-uniform variations in thickness of the elastic lamina of the medial layer and atherosclerotic plaque, at times with hemorrhagic clotting, at the base of the leaflet attachment below its arterial wall (Figure 2).
Figure 2. a-d: frontal histological sections stained with Masson trichrome (a, b, and c) and picrosirius red (d) through the aortic sinuses of the right coronary artery (a, c) and left (b, d). It can be seen that the right and left aortic sinuses have more connective tissue fibers (arrows) in the area where the aortic leaflets attach. The pathological leaflets show atherosclerotic plaque (arrowheads) below the arterial wall of the leaflet. In figure d, under polarized light, the difference in staining can be seen between type I collagen fibers (red-yellow) and those of type III collagen (green). LCA indicates left coronary artery; RAS, right aortic sinus; LAS, left aortic sinus.
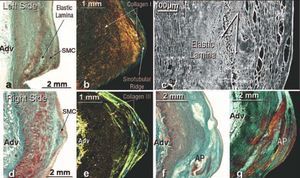
The upper limit of each sinus at the peak of the line of the semicircular edge of each leaflet is known anatomically as the supravalvular ridge, marking the junction between the sinuses and the tubular part of the aorta. The ridge at the sinotubular junction is mainly made up of elastic and collagenous fibers mixed with smooth muscle cells and fibroblasts. The ridge in the left coronary sinus contains a greater number of smooth muscle cells within a dense extracellular matrix of type I collagenous fibers (Figure 3). In contrast, the right coronary artery has a smaller amount of smooth muscle fibers, which are basically set within type III collagen (Figure 3). The aortic wall thickness at the ridge is 4.3±0.5 mm (range, 3.6-5.1 mm), with significant differences between the thickness of each sinus of Valsalva wall and the supravalvular ridge in both coronary arteries (P<.001).
Figure 3. Frontal histological sections stained with Masson trichrome (a, d, f), picrosirius red under polarized light (b, and g) and electron scanning (c) of the sinotubular ridge and left periostial aortic wall (ac) and right (d-g). Note that the sinotubular ridge of the left coronary artery (a, b) has a greater number of smooth muscle cells and type I collagen fibers under polarized light (red-yellow in b) than the right coronary artery (d, e). Under scanning electron microscopy, we can see that the smooth muscle cells (SMC) in the left sinotubular ridge (c) overlap within a dense extracellular matrix, strengthening this part of the coronary ostium. In (f) atherosclerotic plaque (AP) can be seen in the sinotubular ridge of a right coronary artery that affects the tunica media, causing thinning of the aortic wall. In (g) atherosclerotic plaque (AP) appears like layers of an onion under polarized light. Adv indicates tunica adventitia.
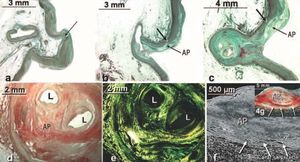
The periostial aortic wall in the sinotubular ridge is characterized by having a prominent tunica media between the internal elastic lamina and the adventitia. This media is predominantly made up of layers of elastic material that alternate with bundles of smooth muscle cells with differing spatial orientation and type I and III collagen fibers (Figure 3). The periostial aortic wall of the right coronary artery has less interstitial type I collagen positivity than the left among the smooth muscle fibers (Figure 3). The thickness of the aortic tunica media in this location was 2.8±0.4 mm (range, 2.1-3.5 mm). The spatial orientation of the smooth muscle cells within the tunica media that surrounds the ostium in both coronary arteries is very irregular, the longitudinal fibers being mixed with oblique ones. The presence of atherosclerotic plaque and intramural hematoma in the sinotubular ridge produces a thinning of the aortic tunica media (Figure 3), less than 1 mm thick, and its visualization via polarized light shows a non-homogeneous distribution of type I collagen within the sinotubular ridge, like layers of an onion, which decreases on the periostial aortic wall (Figure 3). In one case of progressive atherosclerosis, as found in the 72 year old specimen (Figure 4), the aortic tunica media was characterized by an absence of smooth muscle fibers, immediately above the sinotubular ridge, such that the media was made up of elastic fibers only in this region (Figure 4).
Figure 4. Frontal histological sections stained with Masson trichrome (a, b, c) of the sinotubular ridge and periostial aortic wall of the right coronary artery in patients with structural ischemic heart disease. Note in (a) the loss of smooth muscle fibers (arrow) on the aortic wall above the sinotubular ridge. In (b and c) changes can be seen in the aortic tunica media (arrow) which is continuous with the coronary artery tunica media, due to atherosclerotic plaque (AP) and coronary obstruction. In (d and e) cross-sections are shown of the proximal part of an almost obstructed right coronary artery with picrosirius red (d) and polarized light (e). f and g: cross-sections of the proximal part of atherosclerotic plaque (AP) calcified in the left coronary artery with scanning electron microscopy (f) and polarized light (g). Note the atrophy of the arterial tunica media (arrows). L indicates vessel lumen.
In addition to aortic disease, atherosclerotic plaque affects the most proximal segment of the coronary arteries and is accompanied by marked atrophy of the tunica media with a reduction in elastic and smooth muscle fibers, and at times ulceration, i.e., rupture of the plaque coating due to an increase in pressure promoting thrombosis and coronary obstruction. Such obstruction shows positive staining under polarized light, basically for interstitial type I collagen in the adventitia and media (Figure 4). Finally, it is worth considering the possibility that the plaque is sclerosed and calcified (Figure 4), and the percentage reductio n of the lumen is relevant regarding its functional impact.
The most external layer of the aortic coronary wall is the so-called tunica adventitia, which consists of a network of fibers, basically type I collagen, elastic fibers, adipocytes and macrophages (Figures 1 and 2). No visible alterations in this layer were found in the atherosclerotic arteries studied. The vasa vasorum is normally located in the adventitia, where nerve bundles are also found. The thickness of the tunica adventitia in the aortic wall is 1.2±0.4 mm (range, 0.5-1.8 mm). The aortic tunica adventitia is continuous with the adventitia of both coronary arteries.
DISCUSSION
Although the risk of retrogressive dissection in the ascending aorta during PTCA is rare, and that this technique is currently very common and the number of cases has increased, the number of times this serious complication occurs continues to be low. The incidence (0.029%) in our hospital is similar to that in other hospitals, ranging from 0.02 to 0.15%, with an average of 0.059%.1,5,8-9 The mechanism by which dissection of the right coronary artery (87% of cases) occurs more frequently than the left (13% of cases) is not obvious.1,2,5,7-11 Anatomical studies of this area have focused on morphometric and topographical aspects of the coronary artery ostia and have correlated them with aortic leaflets.12-14 However, there are very few structural studies of the relationship between these ostia, the sinuses, and aortic wall.15 The histological and structural study done shows that, of the 30 ostia, 75% of the right coronary artery ostia and 85% of the left coronary artery ostia are located below the sinotubular junction. These results are similar to those obtained by Muriago et al,12 but differ from those contributed by Cavalcanti et al,13 who only found 55% of the coronary ostia below the junction. Our results also agree with the morphological study by Cavalcanti et al,13 who found that the diameter of the ostium of the left coronary artery was larger than that of the right in 76% of their cases. Furthermore, we found that the proximal course of the coronary arteries varies greatly over its initial tract; however, the angle that the left coronary artery forms with the ascending aorta is acute (range, 20o-55o) and that of the right coronary artery tends to be straight (range, 60o-88o). This means that the aortocoronary junction and the proximal course of the left coronary artery can provide a better approach for catheterization than the right coronary artery, due to their coaxial alignment in relation to the ascending aorta. In at least two-thirds of the cases described, dissection occurs when injecting contrast agents. Thus, these variations in angle might play a facilitating role, together with the structural factors, given that the sinotubular junction and the periostial wall of the left coronary artery are different from those of the right. Histologically, although the walls of the right and left sinuses of Valsalva have mainly type I collagen fibers proximal to where the aortic leaflets attach, these fibers decrease in number as the elastic fibers in the ascending part of the aortic sinuses increase; however, higher up, in the sinotubular ridge, the left has a greater number of smooth muscle cells set in large amounts of type I collagen, and its periostial wall also has greater expression of type I collagen. It is well-known that type I collagen, in contrast to type III, has greater tensile strength,16 which could mean that the right coronary artery ostium is less resistant to traction and, as a result, could more easily give rise to retrogressive aortic dissection as a complication of coronary intervention. We have also found structural differences between the normal coronary sinuses and sinotubular junction regarding the specimens with structural heart disease due to myocardial ischemia, which could be a risk factor for increased predisposition to aortocoronary dissection; however, these differences were not found when comparing the atherosclerotic right coronary artery with the left, which indicates that the pathogenesis of the atherosclerotic plaque should be thought of as a set of noxious conditions able to cause endothelial damage, regardless of whether the aortocoronary junction is the right or left although, as stated by Zamir and Sinclair,17 it is the aortocoronary junction which is compromised more often. Although one specimen varies from another, the atherosclerotic lesion penetrates the internal elastic lamina and not only affects the distribution of the sinotubular union type I collagen, but there is also thinning of the smooth muscle fibers of the aortic tunica media. In more severe cases, the weakness of the wall occurs in a 1 or 2 mm longitudinal section in the tunica media, where the smooth muscle fibers are replaced by elastic fibers. Aortic weakness in atherosclerotic disease could be a preexisting factor that may play a role in iatrogenic dissection in the face of aggressive interventions such as mechanical traction or contrast-agent injection, as done during PTCA. Another potential risk factor is the presence of total or partial coronary occlusion in its proximal part, as found in some of the specimens, which possibly plays a role radically different to that of degeneration of the aortic tunica media, since percutaneous recanalization is much more complex and requires more aggressive maneuvers than other types of stenosis.
CONCLUSIONS
Our study demonstrates structural differences between the aortic sinuses and the proximal part of the right and left coronary arteries. These differences indicate that the left aortic sinus is more resistant to traction and mechanical pressure than the right and, thus, is less prone to iatrogenic dissection. Atherosclerotic lesions that compromise the aortocoronary junction are a risk factor that increases predisposition to dissection and should be taken into account during PTCA.
This study was supported by grant SAF 2004-06864 from the Ministry of Education and Science (DS-Q and VC) and the Royal Brompton and Harefield Hospital Charitable Fund (SYH).
Correspondence: Dr. D. Sánchez-Quintana.
Departamento de Anatomía Humana. Facultad de Medicina.
Universidad de Extremadura.
Avda. Elvas, s/n. 06071 Badajoz. España.
E-mail: damians@unex.es
Received November 11, 2005.
Accepted for publication March 23, 2006.