INTRODUCTION
Health registries are useful in determining the extent to which the outcomes of clinical trials and recommendations in clinical guidelines are actually incorporated into medical practice. Conclusions drawn from information in registries can lead to improved prevention and treatment strategies and enhanced resource-allocation, as well as generating new research questions.
The Electrophysiology and Arrhythmia Section (EAS) of the Spanish Society of Cardiology (SSC) set up the first National Registry of Implantable Cardioverter Defibrillators (ICD) in 1996. The first results were published in 1997.1 The EAS's Working Group on Implantable Cardioverter Defibrillators (WGICD) was established in 2001, and last year published its first official report of findings from the Spanish Registry of Implantable Cardioverter Defibrillators for the period 2002-2004.2 The present report provides data on ICD implants collected by the Registry in 2005. Most of the health care centers providing ICD implants in Spain are included in the Registry.
Methodology
Data collection was primarily prospective. Centers used a standard data collection form, which is available on the EAS web-page (www.arritmias.org). ICD implant teams completed this form on a voluntary basis, either during or after the implant, and in collaboration with ICD manufacturers. The completed forms were sent by fax or e-mail to the SCS. In June 2006, sites which had provided prospective data were sent a list of the implants they had reported in 2005. The list included patient initials, the date of the implant, the name of the manufacturer, and the model of implant used. Centers were asked to send retrospective data on any patient who had received an implant but for whom data had not been reported prospectively. Retrospective and prospective data were collected using the same format, and both were communicated by fax or e-mail.
Data was entered into the Spanish ICD Registry database by EAS staff and members of the ICD Working Group. An EAS computer specialist and a member of the ICD Working Group cleaned the data, and members of the current ICD Working Group executive committee were responsible for data analysis and publication.
Population data used to calculate rates per million inhabitants, both nationally and by Autonomous Community and province, were retrieved from the January 1, 2005 estimates of the National Institute of Statistics database (http://www.ine.es).
We used information on the total number of ICD implants or replacements performed in Spain provided by ICD vendors to the European Medical Technology Industry Association (EUCOMED) to calculate the proportion of implants reported to the Registry. This in turn allowed us to estimate the representativity of the Registry.
Where different medical conditions or clinical arrhythmias were reported for the same patient, only the most serious condition was included for analysis.
For each variable analysed, unless otherwise stated, percentages were calculated based on the total number of implants, when that information was available.
Statistical Analysis
Results were expressed as means and standard deviations (SD) in the case of variables with a normal distribution, and as medians and inter-quartile ranges where the distribution was non-normal. Relationships between quantitative variables were analysed using a linear regression model. Qualitative variables were compared using the χ² test. P-values less than .05 were considered statistically significant. Statistical analysis was conducted using the SPSS Inc. Program, version 12.0, Chicago, Ill, USA.
RESULTS
Response rates for the different fields of the data collection form were high, ranging between 70.6% and 98.8% for the Registry's principal variables.
Participating Centers
A total of 79 centers which performed ICD implants provided data to he Registry (table 1). Most of these (n=64) were public health care centers. Table 2 shows the number of public health care centers which provided data to the Registry per million inhabitants and by Autonomous Region.
Total number of implants 2050 (first implants and replacements) were reported to the Registry in 2005 by 29 centers. Of the total number of implants, 1574 (77%) were reported prospectively and 476 (23%) were reported retrospectively. Comparing this total with the manufacturers' figures on the number of implants fitted in 2005, the Registry's figures represent 74.4% of all implants fitted in Spain that year.
The total number of implants per million inhabitants was 46.4 as reported to the Registry and 62.5 according to EUCOMED figures. Table 1 shows the number of implants reported to the Registry by implant center. Table 3 shows the number of implants by Autonomous Community, as reported to the Registry in 2005, and the number of implants per million inhabitants. There was a significant correlation between the number of implant centers per million inhabitants and the number of ICDs fitted in each Autonomous Community (r²=0.59; P=.01).
Table 4 shows the number of implants fitted by province and Autonomous Community, and the figure per million inhabitants.
The majority of implants (1959, or 96% of the implants reported to the Registry) were performed in public health care centers.
First Implants Versus Replacements
There were a total of 1400 first implants (70.3% of all implants fitted) giving a rate of 32 per million. The remaining 593 implants (29.8%) were replacements.
Age and Sex
The mean (SD) age of patients receiving an ICD was 61.8 (14) years (range, 4-90 years). The median (IQR) age was 65 (55-72) years. The figures were very similar for first implants (mean age of 61.6 [13.7] years with the same range). The median age in this group was also the same, with an IQR of 54-72 years. The majority of patients were male (85% of the total and 84.4% of first implants).
Underlying Heart Disease, Left Ventricular Ejection Fraction (LVEF), and Baseline Rhythm
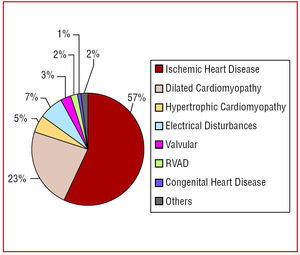
Figure 1 and table 5 show the proportions of different underlying heart disease in patients receiving implants, overall and by first implant. There were no differences in the percentages of underlying heart diseases between first implants and overall. The most frequent underlying condition was ischemic heart disease, followed by dilated cardiomyopathy and hypertrophic cardiomyopathy. An appreciable number of ICDs were performed because of primary electrical disturbances, particularly Brugada syndrome, idiopathic ventricular fibrillation, and long QT syndrome.
Figure 1. Underlying heart disease as reported to the Registry.
The majority of patients had a left ventricle ejection fraction (LVEF) of <30% or 30%-40% (Figure 2). The proportion of patients with an ejection fraction >50% was higher than that of patients with only mild dysfunction (40%-49%). There was a higher proportion of patients with severe left ventricle dysfunction (ejection fraction <30%) in the group of first implants compared to total implants (P<.05).
Figure 2. Left ventricular ejection fraction (LVEF) of patients in the Registry, by total and first implants.
The majority of patients were in functional class I or II in the New York Heart Association classification. There was a lower proportion of patients in functional class III and very few cases of patients in functional class IV (Figure 3).
Figure 3. Functional class (FC) of patients in the Registry, by total and first implants.
The majority of patients (82.3%) presented a sinus rhythm, whereas 12.8% had atrial fibrillation, 4.8% had a pacemaker rhythm, and 8 patients presented other rhythms (atrial flutter or other atrial arrhythmias).
Reason for Implant (Clinical Arrhythmia), Presentation, and Laboratory-Induced Arrhythmia
The leading cause for implant was sustained monomorphic ventricular tachycardia (SMVT), with syncope being the main clinical symptom. The second most frequent cause for implant was non-sustained ventricular tachycardia or ventricular tachycardia without documented clinical arrhythmia. In patients with ventricular tachycardia, ICD was indicated as a preventive measure. The most frequent form of clinical presentation was syncope, followed by "other symptoms," and cardiac arrest. In electrophysiological analysis, SMVT was the most frequently induced arrhythmia in both groups. The proportion of patients without clinical arrhythmia or with non-sustained, asymptomatic ventricular tachycardia was higher in first-implant patients than in the overall group. The proportion of patients in whom no electrophysiological analysis was conducted, or who did not have induced ventricular arrhythmias was also higher among first-implant patients (P<.05 in the 3 comparisons).
Indications (Table 5 and Figure 7)
Figure 4. Clinical arrhythmia of patients in the Registry, by total and first implants. VF/DVT: ventricular fibrillation/deep venous thrombosis; SMVT: sustained monomorphic ventricular tachycardia; SNVT: non-sustained ventricular tachycardia.
Figure 5. Clinical symptoms of arrhythmia presented by patients in the Registry (first implants and total implants). SCD: sudden cardiac death.
Figure 6. Induced arrhythmia in patients in the Registry (first implants and total implants). Cases where no analysis was conducted or where no ventricular arrhythmia was induced are not included. VF/DVT: ventricular fibrillation/deep venous thrombosis; SMVT: sustained monomorphic ventricular tachycardia; SNVT: non-sustained ventricular tachycardia.
Figure 7. Changes in the principal indications for ICD (first implants) 2002 - 2005. SD: Aborted suden death; PROP: prophylactic indication; SMVT: sustained monomorphic ventricular tachycardia; Syncope: syncope without documented electrocardiographic evidence of arrhythmia.
The most frequent indication for ICD was secondary prevention in patients with ischemic heart disease, principally SMVT. The next most frequent indication was prophylactic use in patients with ischemic heart disease. This was most commonly of the MADIT II and COMPANION types.
In patients with dilated cardiomyopathy, the most frequent indication was primary prevention, particularly of the COMPANION type, and to a lesser extent SCD-Heft. These were followed by secondary prevention of SMVT and syncope without documented clinical arrhythmia.
Setting and Personnel
Data on the setting for the operation and the personnel involved was available for 2014 patients. In almost two-thirds of cases, the implant was performed in the electrophysiology laboratory (64.9%), and in 34.9% of cases on the surgical ward. There were isolated cases of implants fitted in other settings.
Implants were primarily performed by electrophysiologists, who carried out 66.4% of the implants. In 26.1% of cases, the device was fitted by a heart surgeon, and in 3.4% of cases the operation was performed jointly by a surgeon and an electrophysiologist. The latter mostly involved fitting of heart resynchronisation devices. Other specialists performed the remaining 4.1% of implants.
Positioning of the Generator
In the great majority of cases, the generator was implanted in a subcutaneous pectoral position (86.3% of total implants and 90.7% of first implants). A sub-muscular pectoral position was used in 12.9% of implants overall and in 9.2% of first implants. Abdominal implants constituted only 0.7% of all implants, and almost all of these were replacements. There was only one case, of an adolescent female, where a first implant was fitted abdominally.
Device Type
55.8% of the devices fitted were si ngle-chamber ICDs, 20.6% were double-chamber ICDs and 23.6% were cardiac resynchronisation therapy (CRT) devices. Double-chamber ICDs represented 27% of the implants without CRT. In the case of first implants, percentages of single-chamber ICDs, double-chamber ICDs and those with CRT were 56%, 20.6% and 23.4%, respectively. Double-chamber ICDs constituted 26.8% of ICDs without CRT. Finally, for replacement ICDs the percentages were 52.5%, 20% and 22.5% respectively. Double-chamber ICDs represented 25.8% of replacements without CRT. It is likely that the majority of the CRT devices recorded in the registry as replacements were updates of previous ICDs in which this function was lacking. According to the 2005 EUCOMED figures, 1495 single-chamber ICDs were fitted (54%), 558 double-chamber ones (20.4%), and 703 with CRT (25.5%).
Reason for Replacement. Substitution of Electrodes in Replacement Generators and Use of Additional Electrodes
In 52.7% of cases, replacements were performed due to battery depletion, and 14.9% were required because of complications. Of a total of 198 cases (33.45%) for which no information was provided, 58 were ICDs with CRT. The majority of these were likely to be updates of earlier ICDS which lacked this function. Among the replacements stemming from complications (n=88), 8 occurred in the 6 months after implant and 65 in the following 6 months. In 15 cases, information was not available. Among the replacements stemming from complications, 9 were prompted by possible device failure, 4 by broken electrodes, 2 because of positioning, and 1 because of endocarditis.
In 80% of cases (n=472), information was available on the prior functioning of the electrodes. 10.8% (n=52) of electrodes were non-functioning. In 41 cases of non-functioning electrodes (79%), the device was removed, though it was maintained in the other 11 cases. Additional defibrillation electrodes were used in 5 cases and sensory electrodes in 3 cases.
Programming of ICDs
Antibradycardia pacing was primarily in VVI mode (54%), with VVIR mode being used in 10.8% of cases, DDD in 20.4%, DDDR in 13.1%, and other pacing methods in 1.7% of cases.
The device was programmed for antitachycardia pacing in 90% of cases, with a combination of ventricular and atrial pacing in 3% of cases. Antitachycardia pacing was not programmed in 7% of cases.
Both ventricular and atrial defibrillation or cardioversion therapies were programmed in 1.8% of cases.
Complications
There were no deaths or cases of cardiac tamponade during the implant operation. Five cases of pneumothorax were reported. Complications arose in 14 implants: these included high defibrillation thresholds (n=2), haematoma or haemorrhage (n=2), acute pulmonary oedema (n=1), electrical storm (n=2), problems of capture (n=1), and coronary sinus dissection (n=1). The remaining complications were not specified.
DISCUSSION
The ICD Working Group within the SCS's Electrophysiology and Arrhythmia Section (EAS) continues to consolidate and improve the Spanish ICD Registry. In 2005, the percentage of information sent prospectively was slightly lower than the previous year, probably because of the initiative to allow centers to send information retrospectively using the same forms as those used to provide information prospectively. Nevertheless, although there was a relative reduction in the amount of information received prospectively, the Registry's representativity improved from 57% to 74.4% which is the highest percentage achieved to date.
Comparison With Previous Years
The number of centers performing implants remained unchanged from the previous year, although the total number of implants reported increased from 1414 to 2050. The number of implants reported per million inhabitants, including both first implants and replacements, increased from 33 to 46.4. The increase was mainly due to the increased number of implants performed. According to EUCOMED data, the number of implants per million inhabitants increased from 52 in 2004 to 62.5 in 2005. The introduction of retrospective reporting of implants which was introduced in 2005, as well as increasing the Registry's representativity, may also have contributed to a smaller degree to the increase in implants reported in 2005.
In terms of indications, the period 2002-2004 saw a significant increase in the total number and proportion of prophylactic implants, due to the publication of several studies which demonstrated the usefulness of ICDs as a preventive measure.3-6 This trend increased in 2005, a year in which the prophylactic use of implants became even better established. Indeed, prophylactic indications were the primary cause of first implants in patients with dilated cardiomyopathy and one of the main causes in patients with ischemic heart disease. The increase in the number of ICDs with CRT is particularly significant.
Lastly, there was a continuation of the trend towards higher proportions of subcutaneous implants and the fitting of ICDs in electrophysiology laboratories by electrophysiologists.
Comparisons With Registries in Other Countries
Information in the scientific literature about ICD implants in other countries remains scarce. The most recent data on the Danish ICD registry (www.pacemaker.dk) is from 2004. In that year, a total of 414 implants and 142 replacements were performed (81 first implants per million inhabitants). The most frequent type of heart disease was ischemia (50%) followed by dilation (25.1%). The most frequent arrhythmias prompting implants were SMVT (55.3% of cases) and ventricular fibrillation (25.1%). Data on the number and type of prophylactic implants was not available. Double-chamber ICDs were used in 34.8% of cases, and ICD in combination with CRT in 17%, indicating a clear increase from 2003. A similar increase was also observed in Spain.
Portugal maintains a National Registry of Cardiac Electrophysiology which includes data on ICD implants. The most recently published information dates from 2004,7 when the total number of implants reported was 397 (344 first implants). This represented an increase of 33.8% on the previous year. The figure for implants per million inhabitants also rose from 21.6 in 2003 to 34.4 in 2004. The registry does not contain information on indications. In terms of the type of ICD, single-chamber models constituted 63.6% of the total, double-chamber ICDs 18.1%, and ICD with CRT 20.6%. The latter represented a 16% increase from the previous year.
Last year, the Italian ICD Registry published information on the years 2001-2003.8 This registry, which was created in 1997 under the auspices of the Italian Society of Arrhythmology and Cardiac Stimulation (SACS) collects information on 85% of the implants carried out in Italy and is based on data from the European Registry of Implantable Defibrillators (EURID). The number of implants in Italy rose from 2400 in 2001 to 5318 in 2003, representing an increase in the number of implants per million inhabitants from 42.1 to 93.3. The number of implant centres increased from 273 in 2001 to 340 in 2003. The figures, although they date from 2003, are significantly higher than those in Spain. The number of prophylactic implants increased three-fold over the 3 years, from 6.4% of implants in 2001 to 18.2% in 2003. Ventricular tachycardia was the underlying cause of implant in 55% of cases, ventricular fibrillation in 18.1%, and both in 6.5%. The percentages of single-chamber ICDs, double-chamber ICDs, and ICD with CRT were 39.2%, 32.4%, and 28.4%, respectively. The figures for ICD with CRT and double-chamber devices, although dating from 2003, were higher than the 2005 Spanish figures.
Data from EUCOMED (the association of the ICD manufacturers) brings together information from Germany, Italy, Ireland, the Netherlands, Denmark, Belgium, Austria, Switzerland, Finland, France, Britain, Spain, Sweden, Portugal, and Norway (Norway did not send data for 2005). In 2005, the mean rate of implants of ICDs with or without CRT in participating countries was slightly over 130 per million. Germany had the highest rate (225) per million, Italy had 190, Ireland and Holland. Denmark, Austria, Belgium, and Switzerland were below the average, but still had over 100 implants per million inhabitants. France had slightly over 80 implants per million. The United Kingdom and Sweden had approximately 70 implants per million, which was slightly more than Spain, and Finland and Portugal had lower rates per million than Spain.
In the United States, the Bilitch registry, which was first established in 1974, included information on ICDs, but it stopped functioning in 1993 because of financial difficulties.9 In the second half of last year, the American College of Cardiology Foundation and the Heart Rhythm Society set up the National ICD Registry, but it has yet to publish any results. It was considered necessary to set up this Registry after several manufacturers recalled devices due to possible device failure. The Registry is voluntary, is accessed via internet (www.accncdr.com/webncdr/ICD/De-fault. aspx), and to date only includes implants for primary prevention.
Geographic Distribution and Regional Differences
The information in the Spanish ICD Registry highlights geographical differences in the resources available, indications, and the number of ICD implants within Spain and in this sense it should be useful for planning purposes. For instance, there is a significant correlation between the number of centres performing implants in each Autonomous Community and the number of ICDs reported to the Registry.
This conclusion is tentative because the Registry does not receive information on every implant, and regional differences may be in part due to the differences in reporting patterns in the different areas. However, regional differences in the number of implant centers per million inhabitants, combined with the fact that almost all publicly-funded ICD centres in Spain are included in the registry, and the representativity of the Registry (approximately 75%), make it more likely that these regional differences reflect the reali implant patterns in Spain. This regional imbalance has also been highlighted in the CARDIOFORUM report, which showed an appreciable regional imbalance in the use of cardiovascular technology (including ICDs, cardiac resynchronisation devices, and percutaneous interventions within the National Health System. Source: CARDIOFORUM).
Limitations
Although the number of implants reported to the Registry, and its representativity, are now higher than ever, the principal limitation remains the level of participation, which currently stands at about 75%. It is to be hoped that the commitment and interest of the professionals involved in ICD implants, combined with the possibility to send data retrospectively as well as prospectively, will enable the Registry to continue growing and improving.
CONCLUSION
The National ICD Registry for 2005 contains information on three-quarters of the ICD implants performed in Spain, and the data can be considered representative of the scale and indications for the procedure in Spain. The number of implants reported to the Registry continued to increase in 2005, and reached 46.4 implants per million inhabitants. Nevertheless, there are appreciable differences in the number of implants reported to the Registry by region. Although secondary prevention is the main reason for AID implants, the number of prophylactic implants showed a greater increase than in previous years, and they now represent a significant proportion of all implants. The increase in the number of combined ICD and CRT implants is particularly significant. The number of ICDs fitted in electrophysiology laboratories by electrophysiologists continues to increase.
ACKNOWLEDGEMENTS
We would like to thank all of the health professionals involved in ICD implants in Spain who have voluntarily and disinterestedly sent data on implants to the Registry. To the staff of the different ICD manufacturers (Medtronic, Guidant, St. Jude Medical, Biotronik, and Ela Medical), for their help in data collection and in sending forms for a large number of implants to the SCS. To the SCS for its support in maintaining the Registry, especially to D. Gonzalo Justes, D. José María Naranjo, and D. Miguel Ángel Salas.
Correspondence: Dr. R. Peinado Peinado.
Unidad de Arritmias. Servicio de Cardiología.
Hospital Universitario La Paz.
Paseo de la Castellana, 261. 28046 Madrid. España.
E-mail: rpeinado@secardiologia.es