Keywords
INTRODUCTION
In 1967, Donald Ross developed native pulmonary valve autograft for aortic valve replacement, in turn replacing the pulmonary valve with a homograft.1 The Ross procedure has since become one of the most successful techniques for aortic valve replacement in certain groups of patients, whether young adults or children.2,3 Pulmonary autograft uses live tissue from the patient's own pulmonary valve. This means the valve grows as the patient grows and can function indefinitely.4 Moreover, patients do not need permanent anticoagulation. Pulmonary autograft permits central laminar flow2,5 and improves hemodynamic performance so that previous dilation and ventricular hypertrophy recede in most patients3,6.
The original indication for pulmonary autograft was treatment of aortic valve disease not susceptible to repair in young patients with life expectancy >=20 years. This has gradually been extended to other indications.2,7,8
Contraindications for this procedure include coexistence of associated coronary disease, moderate or severe multiple valve pathology, connective tissue disease (genetic or autoimmune) and aortic and pulmonary annulus mismatch.2,8-10
However, worse outcomes have been described in patients with congenital characteristics5,11,12 such as bicuspid aortic valve,12,13 which is the most frequent congenital aortic valve condition. This is characterized by changes in the connective tissue, particularly elastic fiber fragmentation, which can extend to the ascending aorta with cystic necrosis of the media, and changes in smooth muscle cell orientation.14 It is also believed to affect the pulmonary autograft and can cause progressive neoaortic insufficiency,13-15 although more recent studies have not supported this theory.16 Worse outcomes have been described in patients with rheumatic etiology or with inflammatory disease of the aortic root as the underlying process, both of which may affect the live tissue autograft and cause postoperative progressive neoaortic insufficiency.11,12
The objective of this study is to compare a series of Ross procedure patients with congenital and acquired etiologies in a short-term follow-up at our hospital in Córdoba (Spain).
PATIENTS AND METHODS
We enrolled all patients undergoing Ross procedure in our hospital from November 1997 thru November 2001. Patients were divided into 2 groups by etiology: congenital (group I) or acquired (group II). The Ross procedure was performed on 61 patients aged 6-54 years: 44 (72%) male and 17 (28%) female. Congenital etiologies identified in 40 (66%) patients were: rheumatic (14, 23%), endocarditis (2, 3%), degenerative (2, 3%), and other (3, 5%). Twelve patients (20%) had bicuspid aortic valves. The valvular lesions were: aortic stenosis (28%), insufficiency (36%), and stenosis and insufficiency (36%). Four patients (6%) had associated coarctation of the aorta, 5 (8%) had subaortic membrane, and 1 had Shone syndrome.
During preintervention clinical interviews we recorded data on age, gender, previous history of cardiac surgery and New York Heart Association (NYHA) functional class: 23% were in NYHA class I, 43% in class II, and 34% in class III. Twenty patients (33%) had previously undergone operations, 12 (20%) with surgery and 8 (13%) percutaneous surgery. General data on the population appear in Table 1.
Surgical Technique8
All operations were elective. Extracorporal circulation with bicaval cannulation of the aorta was used in all patients. We evaluated the native pulmonary valve by transverse pulmonary arteriotomy and resected the diseased aortic valve and complete aortic root conserving right and left coronary artery buttons either freestanding or attached to the aortic wall. Later, we resectioned the right ventricular outflow tract pulmonary autograft (conserving the left anterior descending artery first septal branch) and implanting it in aortic position as free root tied with interrupted sutures on an annulus of autologous pericardium, anastomosing the coronary buttons to the autograft with running suture, and the autograft to the ascending aorta. We performed aortoplasty in patients with ascending aorta dilation. We carried out right ventricular outflow tract reconstruction with a cryopreserved pulmonary homograft with distal and proximal running sutures.
Echocardiography Protocol
Echocardiography was performed with Acuson Sequoia® equipment with M mode, 2D mode, second harmonic imaging, color Doppler, continuous wave Doppler, and pulsed Doppler. Echocardiographic data were collected pre- and intraoperatively, at 6-, 12-, 24-, 36-, and 48-month follow-ups and on presentation of clinical events (see appendices).
Clinical Follow-up
Patients were invited to attend clinical re-evaluation including NYHA functional status and occurrence of events at 6, 12, 24, 36, and 48 months and on presentation of clinical events. These were defined as all-cause death (including perioperational death), need for reoperation due to autograft dysfunction, need for stent implantation due to homograft stenosis or graft endocarditis. Mean follow-up was 15.6±10.6 months.
Chi-square was used to compare qualitative variables, Student t test to compare quantitative variables, and Kaplan-Meier to construct survival curves.
RESULTS
Average age of patients with congenital etiology (group I) was 24±11 years and 67.5% were males. Average age of patients with acquired etiology (group II) was 40±7 years and 81% were males. Table 2 presents preoperative data by etiology.
Age was significantly lower in group I (P<.001); differences in gender between groups were statistically nonsignificant. Group I patients had fewer preoperative symptoms but the difference was statistically nonsignificant (NYHA functional class I: group I 30.8% vs group II 5.6%; P=.03). Group I patients presented higher incidence of previous cardiac surgery (50% vs 5%; P<.001). Mean left ventricular ejection fraction was normal in both groups (70.7% vs 61.8%). Differences in body surface area adjusted left ventricular dimensions were statistically nonsignificant: left ventricular diastolic diameter (37.5 mm/m2 vs 35.0 mm/m2); left ventricular systolic diameter (21.2 mm/m2 vs 22.4 mm/m2); interventricular septum (7.02 mm/m2 vs 7.5 mm/m2); left ventricular posterior wall (6.05 mm/m2 vs 5.8 mm/m2). Differences in transaortic gradient were statistically nonsignificant: peak gradient 67.9 mm Hg versus 59.6 mm Hg; mean gradient 44.3 mm Hg versus 42.7 mm Hg. In intraoperative data, we found no statistically significant differences for immediate left ventricular ejection fraction and autograft competence (normal: 100% vs 95.2%) and immediate postoperative homograft status (normal: 100% in both groups).
At final follow-up, body surface area adjusted left ventricular dimensions were normal in both groups with statistically nonsignificant differences: left ventricular diastolic diameter (30.7 mm/m2 vs 30.3 mm/m2); left ventricular systolic diameter (19.3 mm/m2 vs 20.2 mm/m2); interventricular septum (6.1 mm/m2 vs 5.3 mm/m2); left ventricular posterior wall (6.6 mm/m2 vs 5.9 mm/m2). Similarly, at follow-up ejection fraction was normal and similar in both groups (64.9% vs 60.2%). Annulus diameters produced similar data for autograft (24.8 mm vs 26.2 mm) and homograft (18.8 mm vs 20.7 mm). At final follow-up, differences between groups for peak autograft gradient (7.8 mm Hg vs 7.2 mm Hg), mean autograft gradient (4.4 mm Hg vs 4.1 mm Hg), and peak homograft gradient (19.9 mm Hg vs 18.1 mm Hg) were statistically nonsignificant. Final follow-up data on seriousness of autograft insufficiency (without insufficiency or with mild insufficiency: 93.4% vs 84.3%), and homograft insufficiency (without insufficiency or with mild insufficiency: 100% in both groups) were statistically nonsignificant (Figure 1). Table 3 shows final follow-up echocardiographic data for both groups.
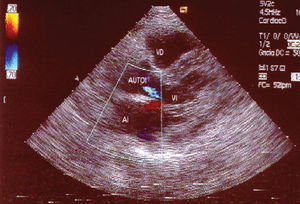
Fig. 1. Parasternal long axis view showing mild autograft regurgitation. AI indicates left atrium; AUTOI, autograft; VD, right ventricle; VI, left ventricle.
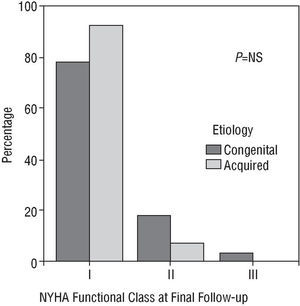
At final follow-up, NYHA functional class showed no statistically significant differences between groups and the majority of patients in both groups were asymptomatic (Figure 2). In group I, 78.6% were in class I, 17.9% in class II, and 3.5% in class III; in group II 92.3% were in class I and 7.7% in class II.
Fig. 2. Comparison of NYHA functional class of both groups at final follow-up. NYHA indicates New York Heart Association.
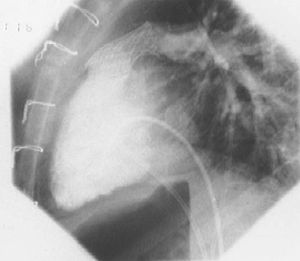
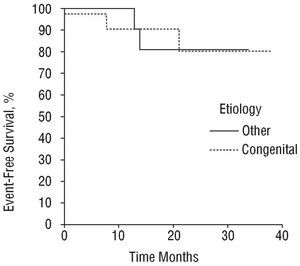
One patient died in hospital (1.6%) (group I). No patient died during follow-up and global final follow-up mortality was 1.6%. Comparison of the total or individual number of "events" did not differ between groups (Table 4): 4 (10%) events in group I (1 patient died; 1 had autograft endocarditis; 2 patients needed stent implantation in the autograft [Figure 3]); and 2 (9.5%) events in group II (2 patients needed autograft surgery). Kaplan-Meier event-free survival curves are similar in both groups (Figure 4) with 81% mean event-free survival at 38 months.
Fig. 3. Pulmonary homograft stent implant.
Fig. 4. Event-free survival curves (Kaplan-Meier curves).
Among patients with congenital etiology (group I), follow-up data in patients with bicuspid aortic valve show no significant difference when compared with patients with tricuspid aortic valve. Degree of autograft insufficiency (patients with bicuspid aortic valve presented 100% without or with mild insufficiency vs patients with tricuspid aortic valve 89.5%). Peak autograft gradient (patients with bicuspid aortic valve 6.8 mm Hg vs patients with tricuspid aortic valve 8.6 mm Hg); mean autograft gradient (patients with bicuspid aortic valve 3.6 mm Hg vs patients with tricuspid aortic valve 4.7 mm Hg) and peak homograft gradient (patients with bicuspid aortic valve 20.3 mm Hg vs patients with tricuspid aortic valve 19.8 mm Hg) were similar in the 2 subgroups at final follow-up. Two patients in each subgroup experienced a clinical event during follow-up: 1 patient with bicuspid aortic valve died and 1 needed stent implantation in the homograft; 1 patient with tricuspid aortic valve had endocarditis and 1 needed stent implantation in the homograft. Differences between these subgroups were statistically nonsignificant.
Short-term follow-up results of the series of patients undergoing Ross procedure in our hospital show low mortality and morbidity, independent of etiology.
The Ross procedure is a complex aortic valve replacement operation used with great success in patients with congenital and acquired etiologies.2,3,5,13 Among possible complications found at follow-up we identified autograft insufficiency and progressive homograft stenosis and found stent implantation in the graft is sometimes needed.17 Xie et al5 compared young (<40 years) and older patients (≥40 years) without differentiating between etiologies and found that homograft stenosis is less common and less serious in older patients, perhaps due to calcium turnover and reduced immune response status. Progressive neoaortic insufficiency in the young rheumatic population has been described in Ross procedure patients11 and worse evolution has been identified in those with bicuspid aortic valve15,16 due to degeneration of the ascending aorta and secondary autograft insufficiency.
The present series distinguished between patients with congenital and acquired aortic pathologies because data in the literature are contradictory about results in these groups. In our evaluation of pre- and postoperative data, echocardiography provides measures of left and right ventricular size and function, valve annulus or graft annulus size, graft competence and transvalvular gradients. Echocardiography also identifies complications such as endocarditis and can indicate need for reoperation or stent implantation due to homograft stenosis. Consequently, echocardiography is as essential to pre- and postoperative study of Ross procedure patients18 as the clinical history and clinical follow-up.
The majority of patients in both groups were in NYHA class I at final follow-up. This corresponds to other international series in which patients show low follow-up morbidity.5
Although the Ross procedure is technically more complex, inhospital mortality was 1.6%. This is very low by comparison with other aortic valve replacement procedures that have inhospital mortality rates of 10%.19 Events (death, need for surgery, need for stent implantation or endocarditis) are equally frequent in the two groups: The only patient who died was in the congenital etiology group, had previously undergone surgery and had a complex congenital heart condition (Shone syndrome). This patient's death may have been more closely related to the associated condition than the etiology of the valve lesion. Patients with congenital aortic valve disease were younger and this may explain the concentration of stent implants due to homograft stenosis in this group, even though differences were statistically nonsignificant. We found no cases of homograft endocarditis. The one case of late autograft endocarditis occurred in a patient with congenital etiology, who presented moderate-severe autograft insufficiency. Again, differences between the two groups were statistically nonsignificant.
The need for reoperation of the autograft seems to have been concentrated in the group of patients with acquired etiology, although the difference was statistically nonsignificant. Earlier studies report development of progressive neoaortic insufficiency in patients with rheumatic etiology.11 In our series, 2 patients with rheumatic etiology required autograft surgery for severe aortic insufficiency. These were two of the earliest patients in the series and technical inadequacies of the procedure may have been the cause as autograft reinforcement with pericardial annulus was not used and dysfunction occurred early in the first month. We have had no cases of severe aortic insufficiency in later operations. However, rheumatic conditions are not studied in isolation but classified as degenerative disease or postendocarditis.
Allegedly poor mid-term results of the Ross procedure in patients with bicuspid aortic valve13,16 were not evident in our series. At short-term follow-up, differences between patients with bicuspid aortic valve and patients with tricuspid aortic valve were statistically nonsignificant although a longer-term follow-up is needed to determine whether differences do exist. We coincide with other series that report excellent mid-term outcomes in patients with bicuspid aortic valve.20 Some authors have described worse evolution of the autograft in patients with bicuspid aortic valve when the previous lesion type is aortic regurgitation rather than aortic stenosis.21 This is not supported by our series as short-term autograft dysfunction has not occurred in the subgroup of patients with bicuspid aortic valve.
The limitation of this study is the low incidence of events, which makes it difficult to analyze and limits its value statistically. However, the fact that morbidity and mortality are low is positive when electing the Ross procedure for young patients with aortic disease requiring valve replacement, regardless of the etiology.
We conclude that hemodynamic performance and short-term evolution of patients who undergo the Ross procedure for aortic valve disorders are similar whether patients have congenital or acquired conditions. Moreover, in patients with congenital etiology whether the aortic valve is bicuspid or tricuspid has no apparent influence on these parameters. Consequently, unless contraindicated, the procedure seems adequate in aortic valve disease of any etiology.
Correspondence: Dra. C. Rus Mansilla.
Juan J. Cuenca, 25, 2.o A. 14900 Lucena. Córdoba. España.
E-mail: crusmansilla@yahoo.es