Beta-blockers are the cornerstone of treatment for various cardiovascular conditions. Although their effects have classically been considered to be driven by their antagonistic and competitive action on beta-adrenergic receptors, nowadays it is known that their effect goes beyond that of mere competition with catecholamines on these receptors. Beta-blockers were discovered as antianginal drugs in the 1960s and are currently widely used in heart failure, arrhythmias, and ischemic heart disease. In this article, we review the evidence for the beneficial effects of beta-blockers in these conditions, as well as the current recommendations in clinical practice guidelines for their use. Surprisingly, despite having been prescribed for more than 4 decades, new, previously unnoticed mechanisms of action on cellular compartments are still being discovered, which continues to open up new horizons for their use. All in all, beta-blockers are one of the most fascinating drug groups in our therapeutic armamentarium.
Keywords
Few drug groups have been as widely studied as beta-blockers (BBs) in patients with different cardiovascular conditionsover the years. In the last 3 decades, they have revolutionized the field of cardiology, and extensive evidence corroborates their efficacy in the 4 most common groups of cardiovascular diseases: heart failure (HF), hypertension, arrhythmias, and ischemic heart disease. At the same time, new mechanisms of action of BBs are still being found, which help to better explain the reasons for their clear benefits.1 These new discoveries further expand the potential, but as yet, unidentified applications of BBs in clinical settings.
Here, we present the existing evidence on the benefits of BBs in different clinical contexts, the mechanisms of action underlying these benefits, and the current recommendations.
BETA-BLOCKERS IN HEART FAILUREDue to their negative inotropic properties, BBs were long considered absolutely contraindicated in patients with HF. The field underwent a conceptual revolution upon considering that, contrary to the prevailing wisdom, BBs could paradoxically be beneficial. In the first decade of the 21st century, these drugs were shown to have highly positive effects in patients with HF. Since then, they have become a cornerstone in the treatment of HF in patients with systolic dysfunction (reduced left ventricular ejection fraction [LVEF], ≤ 40%; also known as reduced ejection fraction [rEF]). However, HF must be understood in its entire spectrum, from asymptomatic patients who are nonetheless at risk for HF—stage A of the American College of Cardiology/American Heart Association (ACC/AHA)2—to symptomatic patients with different LVEF ranges and even hospitalized patients or those with severe HF symptoms.
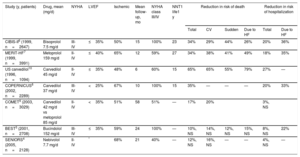
Heart failure with reduced ejection fractionThe evidence conclusively shows that BBs reduce the risk of death in patients with HFrEF. Their use is widely recognized in the recent clinical practice guidelines of the European Society of Cardiology (ESC).3Table 1 shows data from the main randomized trials4-10 supporting the use of BBs, as well as their ability to reduce the most important endpoints. Although metoprolol, bisoprolol, and carvedilol are associated with significant beneficial effects, the association is not as consistent for bucindolol and nebivolol. In the SENIORS trial (age > 70 years, 64% with rEF), nebivolol was associated with a lower risk of the composite endpoint of death and cardiovascular hospitalization, but not of death alone or the other endpoints.4 Bucindolol failed to reduce mortality in the BEST study, although it did reduce hospitalizations for HF.5 The 3 BBs with the strongest evidence in this population (metoprolol, bisoprolol, and carvedilol) are associated with reduced disease progression, as shown by the lower rates of directly related death (sudden and HF) and HF hospitalizations in the various trials.6–8
Design and results of the main clinical trials of beta-blockers in heart failure
| Study (y, patients) | Drug, mean (mg/d) | NYHA | LVEF | Ischemic | Mean follow-up, mo | NYHA class III/IV | NNT1 life1 y | Reduction in risk of death | Reduction in risk of hospitalization | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | CV | Sudden | Due to HF | Total | Due to HF | ||||||||
| CIBIS-II6 (1999, n=2647) | Bisoprolol 7.5 mg/d | III-IV | ≤35% | 50% | 15 | 100% | 23 | 34% | 29% | 44% | 26% | 20% | 36% |
| MERIT-HF7 (1999, n=3991) | Metoprolol 159 mg/d | II-IV | ≤40% | 65% | 12 | 59% | 27 | 34% | 38% | 41% | 49% | 18% | 35% |
| US carvedilol10 (1996, n=1094) | Carvedilol 45 mg/d | II-IV | ≤35% | 48% | 6 | 60% | 15 | 65% | 65% | 55% | 79% | 27% | — |
| COPERNICUS8 (2002, n=2289) | Carvedilol 37 mg/d | III-IV | <25% | 67% | 10 | 100% | 15 | 35% | — | — | — | 20% | 33% |
| COMET9 (2003, n=3029) | Carvedilol 42 mg/d vs metoprolol 85 mg/d | II-IV | <35% | 51% | 58 | 51% | — | 17% | 20% | 3%, NS | |||
| BEST5 (2001, n=2708) | Bucindolol 152 mg/d | III-IV | ≤35% | 59% | 24 | 100% | — | 10%, NS | 14%, NS | 12%, NS | 15%, NS | 8%, NS | 22% |
| SENIORS4 (2005, n=2128) | Nebivolol 7.7 mg/d | II-IV | * | 68% | 21 | 40% | — | 12%, NS | 16%, NS | — | — | 4%, NS | — |
CV, cardiovascular; HF, heart failure; LVEF, left ventricular ejection fraction; NNT, number needed to treat; NS, not significant; NYHA, New York Heart Association.
All studies analyzed beta-blockers vs placebo, except COMET (carvedilol vs metoprolol tartrate). All risk reductions are significant, unless otherwise indicated.
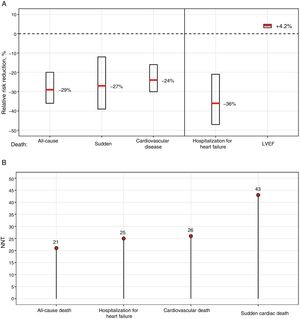
The COMET study, the only trial to directly compare 2 BBs—carvedilol vs metoprolol tartrate—, found lower mortality with carvedilol; however, the use of short-acting metoprolol, contrasting with the formulation used in the MERIT-HF trial,7 might somewhat explain these differences.9 In a large meta-analysis based mainly on BBs with proven survival benefit, no differences were found among the different BBs, which together reduced 12-month mortality by 31% without treatment–subgroup interactions.11Figure 1 shows the relative risk reduction and the number of patients needed to treat at 1 year to reduce the different events, based on the meta-analysis results.11
In recent years, different meta-analyses have addressed the relationship between the benefits of BBs and heart rate in patients with rEF. The connection between a higher heart rate and worse prognosis is well established. Nonetheless, a subanalysis of the HF-ACTION study showed that the benefit is greater in the presence of high BB doses, regardless of heart rate.12 On the other hand, other recent meta-analyses have indicated that the heart rate reduction-related benefit of BBs is only achieved in patients with sinus rhythm or is at least lower in patients with atrial fibrillation (AF).13 However, a subanalysis of the AF-CHF study showed that BBs also improved mortality rates in patients with AF and rEF.14
A particular issue is patients with asymptomatic rEF. In these patients, BBs theoretically prevent the adverse ventricular remodeling processes that promote the progression to symptomatic HF. The REVERT trial, the only study to randomize asymptomatic patients in New York Heart Association (NYHA) stage I and with rEF (stage B of the ACC/AHA),1 showed that metoprolol succinate was associated with reduced volumes and increased LVEF at 1 year.15 The CAPRICORN study (postinfarction LVEF <40%) identified less progression to symptomatic stages and improved remodeling and ventricular function.16 In an observational study, BB use reduced episodes of symptomatic HF by 60%.17
Heart failure with midrange or preserved systolic functionProspective clinical trials are scarce and typically have indirect endpoints such as echocardiographic parameters or small sample sizes that merely allow estimation of the effect on clinical endpoints. An observational study, based on propensity score adjustment with a large population of hospitalized patients, and several meta-analyses indicate that BBs can reduce mortality in patients with a LVEF > 40%.18 Recently, a substudy of the TOPCAT trial (LVEF> 45%) showed that BBs, particularly in patients without previous infarction, were associated with increased adverse cardiovascular events.19 However, after the recommendation in the latest European guidelines to consider patients with midrange LVEF (40%-49%) as a separate subgroup, an individual meta-analysis that included the LVEF of each patient in the pivotal clinical trials showed that patients in sinus rhythm could indeed benefit from BB therapy in terms of mortality.20
Severe acute or decompensated heart failureFor patients hospitalized with rEF, continuation of BBs during admission reduces the risk of death by 40%, whereas initiation of BB therapy in BB-naïve patients reduces the risk by almost 60%.21 In contrast, withdrawal of BBs during hospitalization doubles mortality.22 The COPERNICUS study evaluated patients with severe HF (NYHA class III-IV and LVEF <25%), including hospitalized or decompensated patients taking intravenous diuretics. The use of carvedilol reduced the overall risk of death by 35%.7 In addition, BB initiation during hospitalization facilitated adherence to BB therapy during follow-up.23 In another subanalysis of the MERIT-HF trial that included patients with worse clinical deterioration (NYHA class III-IV and LVEF <25%), the benefit of metoprolol was clear and even greater.24
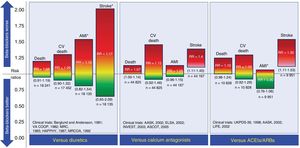
BETA-BLOCKERS IN HYPERTENSIONCompared with placebo, BBs have not been shown to reduce all-cause or cardiovascular mortality in patients with uncomplicated essential hypertension.25–27 However, they can reduce cardiovascular events, mainly stroke. Compared with diuretics, BBs do not reduce cardiovascular events and may even be associated with a higher incidence of stroke.28 This high stroke incidence appears to be associated with age, which increases the risk of BBs in those older than 60 years.29 Compared with calcium antagonists or angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, BBs are associated with an increased risk of stroke.26,27 The results of the use of BBs as first-line drugs in the treatment of hypertension vs other drug groups are shown in Figure 2.
Comparison of beta-blockers with other drugs used as first-line strategies for the treatment of essential hypertension. The risk ratios are plotted together with their 95% confidence intervals (in parentheses). ACEIs, angiotensin-converting enzyme inhibitors; AMI, acute myocardial infarction; ARBs, angiotensin II receptor blockers; CV, cardiovascular; RR, risk ratio. *Events whose risk ratio and corresponding confidence interval have a low level of certainty according to the evidence level classifications of the GRADE working group. The information is based on the meta-analysis by Wiysonge et al.27
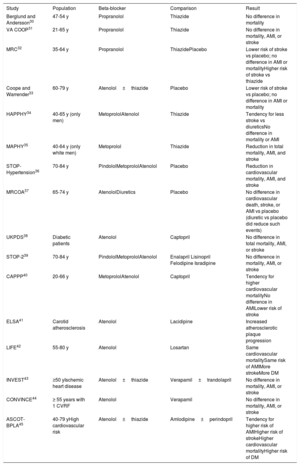
Because BBs are a heterogeneous group of drugs, conclusions derived from meta-analyses should generally be taken with caution. Table 2 shows the results of the most important clinical trials that have analyzed the roles of the different BBs in the treatment of hypertension.30–45
Main clinical studies analyzing beta-blockers in the treatment of essential hypertension
| Study | Population | Beta-blocker | Comparison | Result |
|---|---|---|---|---|
| Berglund and Andersson30 | 47-54 y | Propranolol | Thiazide | No difference in mortality |
| VA COOP31 | 21-65 y | Propranolol | Thiazide | No difference in mortality, AMI, or stroke |
| MRC32 | 35-64 y | Propranolol | ThiazidePlacebo | Lower risk of stroke vs placebo; no difference in AMI or mortalityHigher risk of stroke vs thiazide |
| Coope and Warrender33 | 60-79 y | Atenolol±thiazide | Placebo | Lower risk of stroke vs placebo; no difference in AMI or mortality |
| HAPPHY34 | 40-65 y (only men) | MetoprololAtenolol | Thiazide | Tendency for less stroke vs diureticsNo difference in mortality or AMI |
| MAPHY35 | 40-64 y (only white men) | Metoprolol | Thiazide | Reduction in total mortality, AMI, and stroke |
| STOP-Hypertension36 | 70-84 y | PindololMetoprololAtenolol | Placebo | Reduction in cardiovascular mortality, AMI, and stroke |
| MRCOA37 | 65-74 y | AtenololDiuretics | Placebo | No difference in cardiovascular death, stroke, or AMI vs placebo (diuretic vs placebo did reduce such events) |
| UKPDS38 | Diabetic patients | Atenolol | Captopril | No difference in total mortality, AMI, or stroke |
| STOP-239 | 70-84 y | PindololMetoprololAtenolol | Enalapril Lisinopril Felodipine Isradipine | No difference in mortality, AMI, or stroke |
| CAPPP40 | 20-66 y | MetoprololAtenolol | Captopril | Tendency for higher cardiovascular mortalityNo difference in AMILower risk of stroke |
| ELSA41 | Carotid atherosclerosis | Atenolol | Lacidipine | Increased atherosclerotic plaque progression |
| LIFE42 | 55-80 y | Atenolol | Losartan | Same cardiovascular mortalitySame risk of AMIMore strokeMore DM |
| INVEST43 | ≥50 yIschemic heart disease | Atenolol±thiazide | Verapamil±trandolapril | No difference in mortality, AMI, or stroke |
| CONVINCE44 | ≥ 55 years with 1 CVRF | Atenolol | Verapamil | No difference in mortality, AMI, or stroke |
| ASCOT-BPLA45 | 40-79 yHigh cardiovascular risk | Atenolol±thiazide | Amlodipine±perindopril | Tendency for higher risk of AMIHigher risk of strokeHigher cardiovascular mortalityHigher risk of DM |
AMI, acute myocardial infarction; CVRF, cardiovascular risk factors; DM, diabetes mellitus.
The new European guidelines on hypertension46 rule out BBs as first-line drug therapy for uncomplicated hypertension.
BETA-BLOCKERS AND CARDIAC ARRHYTHMIASβ1-receptors constitute 80% of the adrenergic receptors in the heart. By blocking these receptors, BBs counteract the proarrhythmic effect of sympathetic activity on the myocardium.47 The antiarrhythmic effect of BBs is the result, on the one hand, of its direct cardiac electrophysiological action, which is mediated in different ways: by reducing heart rate, decreasing the spontaneous activation of ectopic pacemakers, slowing down the conduction of electrical impulses, or increasing the refractory period of the atrioventricular node. On the other hand, their antiarrhythmic properties are influenced by other mechanisms that, although not of direct electrophysiological cardiac activity, do help to prevent cardiac arrhythmias, via inhibition of sympathetic activity, reduction of myocardial ischemia, an effect on baroreflex function, and decreased mechanical stress. These effects differentiate BBs from other antiarrhythmics, which exert their activity via direct modulation of cardiomyocyte ion channels. BBs have limited proarrhythmic effects and thus have an excellent efficacy and safety profile.
Atrial fibrillationBBs are first-line drugs for the control of heart rate in the context of AF in patients without contraindications.48 Although rate control therapy plays a fundamental role in AF management, sympathetic activity is related to both AF initiation and maintenance.49 Nonetheless, the role of BBs in rhythm control is secondary, although it is true that, in a randomized study vs placebo, metoprolol reduced AF recurrence by 11%.50 In addition, in patients with HF or acute myocardial infarction (AMI), BBs can reduce the incidence of AF.51
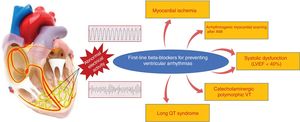
Ventricular arrhythmiasBBs are particularly useful for the control of ventricular arrhythmias related to sympathetic activity, such as perioperative arrhythmias and those associated with stress, AMI, and HF.52 They prevent sudden cardiac death by reducing malignant ventricular arrhythmias in different contexts, mainly under acute ischemic, systolic dysfunction, and channelopathy conditions. In the setting of AMI, BBs increase the threshold for ventricular fibrillation during acute ischemia.53,54 In a more stable phase, they are especially useful for preventing scar-related ventricular arrhythmias after a confirmed AMI, which generally present as sustained monomorphic ventricular tachycardia. In patients with rEF, an absolute reduction in sudden cardiac death rates of about 2% to 3% has been demonstrated (∼40% relative reduction vs placebo).55 In patients with channelopathies, particularly those with long QT syndrome and catecholaminergic ventricular tachycardia, BBs are the drug of choice. In this context, a retrospective study of 233 patients with long QT syndrome and a history of syncope showed a marked reduction in mortality with BBs vs placebo.56 For patients with catecholaminergic polymorphic ventricular tachycardia, BBs are the drug of choice, particularly nadolol.57 The clinical situations with proven benefit of BBs for the prevention of sudden cardiac death due to ventricular arrhythmias are shown in Figure 3.
BETA-BLOCKERS IN ISCHEMIC HEART DISEASEBBs have been used for several decades to manage ischemic heart disease in contexts such as during AMI (intravenous administration), in chronic administration after AMI, or in patients with coronary disease without previous AMI. Multiple studies in the prereperfusion era examined the beneficial effect of BBs in the setting of AMI and showed a clear reduction in long-term mortality.58
Intravenous beta-blockers in the acute phase of infarctionDuring the course of AMI, catecholamine-mediated sympathetic activity occurs in response to pain, anxiety, and decreased cardiac output. This increase in sympathetic tone has negative consequences, such as an elevated myocardial oxygen demand that accelerates myocardial necrosis and a decreased ventricular fibrillation threshold that increases the risk of sudden cardiac death. The increased sympathetic activity also activates various types of circulating cells, such as platelets and neutrophils. The latter significantly contribute to the phenomenon known as reperfusion injury.59
Most trials conducted in the prereperfusion era compared the use of intravenous BBs immediately after AMI diagnosis followed by oral BBs vs placebo.58 The early initiation of intravenous BB was explored as an intervention capable of limiting the extent of necrosis, but the results were inconclusive. In the absence of reperfusion, it is difficult to find a benefit in limiting the extent of the necrosis. In the pharmacological reperfusion (fibrinolysis) era, intravenous atenolol was ineffective in reducing infarct size in a randomized trial.60 However, another nonrandomized study showed that intravenous metoprolol was associated with a smaller infarct size.61 The first clinical study of this issue in patients reperfused by primary angioplasty was the METOCARD-CNIC trial, performed in Spain.62 In this trial, metoprolol administration was associated with smaller infarct size53 and higher long-term LVEF.63 Another subsequent trial, the EARLY-BAMI study, failed to corroborate the cardioprotective effects of early metoprolol administration in patients with AMI undergoing primary angioplasty.64 The discrepancy between the 2 trials seems to be due to the time of metoprolol administration (much earlier in METOCARD-CNIC) because the longer the time between the intravenous administration of metoprolol and reperfusion, the greater its cardioprotective effect.65 The mechanism underlying the ability of metoprolol to reduce infarct size when it is intravenously administered early before reperfusion appears to involve a direct effect on circulating neutrophils and their aggregation with platelets, which results in reduced reperfusion injury and reduced microvascular obstruction.66
The early use of intravenous BBs in AMI has been questioned because of its potential ability to increase the incidence of cardiogenic shock. This concern is based on the results of the COMMIT study.54 In this trial, the early administration of metoprolol to patients with AMI was associated with a significant reduction in ventricular fibrillation, but an increase in shock. However, the COMMIT population included patients with advanced disease. In addition, half did not undergo reperfusion. Moreover, patients who developed cardiogenic shock had clear signs of acute HF along with tachycardia and hypotension. In contrast, a meta-analysis that included all trials involving early administration of intravenous BBs, which amounted to more than 73 000 patients, demonstrated that this strategy is safe if applied to patients without signs of HF and also significantly reduces the frequency of ventricular fibrillation.67
Based on the new trials performed in patients undergoing primary angioplasty, the ESC guidelines for the treatment of patients with ST-segment elevation AMI recommend the early use of intravenous BBs (class IIa A) in the absence of HF signs or systolic hypotension (<120mmHg).68
Maintenance beta-blockers after infarctionThe use of BBs after AMI was exhaustively investigated in the prereperfusion era.58 Apart from the COMMIT trial,54 with a follow-up of only 1 month, the only clinical trial to examine the role of oral maintenance BBs after AMI is the CAPRICORN study.16 In this trial, 1950 post-AMI patients with LVEF ≤ 40% were randomized to carvedilol or placebo. BB use was associated with a reduction in overall mortality.16 Because many of the trials focusing on HFrEF (Table 1) included post-AMI patients, it is considered established that all patients who have had an AMI with a LVEF ≤ 40% have an indication for BBs. However, there is a lack of evidence on the benefits of BBs for post-AMI patients with a LVEF> 40%. Numerous observational studies have attempted to shed light on this issue, but all have major limitations and thus do not provide definitive information (an aspect reviewed by Ibáñez et al.1).
Given all of the above, the ESC clinical practice guidelines on patients with AMI strongly (class IA) recommend BB use whenever LVEF is ≤ 40%, regardless of whether patients have ST-segment or non–ST-segment elevation AMI. However, the recommendation for patients who have had an AMI with a LVEF> 40% is weaker.1
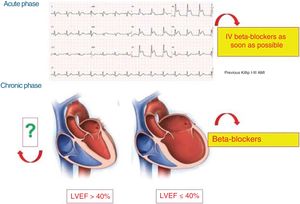
Figure 4 shows the clinical situations with proven benefit of BBs in the context of AMI.
Due to this lack of evidence on such an important aspect of daily clinical care, 3 large pragmatic clinical trials are underway in Europe to explore the role of BBs in patients without reduced LVEF who have had an AMI. The REBOOT clinical trial (NCT03596385), directed from the National Center for Cardiovascular Research (CNIC) in Spain, involves the participation of more than 70 Spanish and Italian centers. About 8500 post-AMI patients with LVEF> 40% will be included in this large Spanish study. In addition, the REDUCE-SWEDEHEART (NCT03278509) and BETAMI (NCT03646357) trials are being conducted in Sweden and Norway, respectively; their designs are similar to that of REBOOT. These large clinical trials will have a clear impact on clinical practice in this setting.
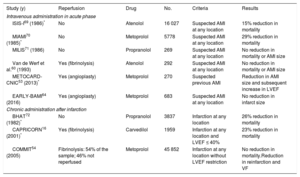
Table 3 summarizes the results of the different clinical trials16,53,54,60,64,69–72 that have analyzed the role of BBs, either in the acute phase of AMI or in the maintenance period after the acute event.
Main trials on the use of beta-blockers in acute coronary syndrome
| Study (y) | Reperfusion | Drug | No. | Criteria | Results |
|---|---|---|---|---|---|
| Intravenous administration in acute phase | |||||
| ISIS-I69 (1986)* | No | Atenolol | 16 027 | Suspected AMI at any location | 15% reduction in mortality |
| MIAMI70 (1985)* | No | Metoprolol | 5778 | Suspected AMI at any location | 29% reduction in mortality |
| MILIS71 (1986) | No | Propranolol | 269 | Suspected AMI at any location | No reduction in mortality or AMI size |
| Van de Werf et al.60 (1993) | Yes (fibrinolysis) | Atenolol | 292 | Suspected AMI at any location | No reduction in mortality or AMI size |
| METOCARD-CNIC53 (2013)* | Yes (angioplasty) | Metoprolol | 270 | Suspected previous AMI | Reduction in AMI size and subsequent increase in LVEF |
| EARLY-BAMI64 (2016) | Yes (angioplasty) | Metoprolol | 683 | Suspected AMI at any location | No reduction in infarct size |
| Chronic administration after infarction | |||||
| BHAT72 (1982)* | No | Propranolol | 3837 | Infarction at any location | 26% reduction in mortality |
| CAPRICORN16 (2001)* | Yes (fibrinolysis) | Carvedilol | 1959 | Infarction at any location and LVEF ≤ 40% | 23% reduction in mortality |
| COMMIT54 (2005) | Fibrinolysis: 54% of the sample; 46% not reperfused | Metoprolol | 45 852 | Infarction at any location without LVEF restriction | No reduction in mortality.Reduction in reinfarction and VF |
AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction; VF, ventricular fibrillation.
The antianginal effects of BBs are well established and are included in the clinical practice guidelines.73 Compared with calcium channel blockers, BBs reduce anginal episodes and the time to ischemia onset in exercise testing.74 However, no clinical trial has studied in a randomized manner and with sufficient statistical power whether BBs improve the survival of patients with stable coronary disease but without AMI or rEF. In a systematic review and meta-analysis, their use did not reduce mortality.75 The REACH registry, which included more than 40 000 Swedish patients, found no benefit in patients with stable coronary disease but without previous AMI after propensity score adjustment. Several studies support the conclusion that, in the presence of stable coronary disease and without previous AMI, BB use does not have beneficial effects on mortality and adverse cardiovascular events.76
CONCLUSIONSBBs are a group of drugs that are part of the standard therapeutic armamentarium for severa cardiovascular conditions. Their benefits in patients with HF and ventricular dysfunction are clearly established, as well as their antiarrhythmic effects. In the context of AMI, early administration of intravenous BBs reduces the incidence of ventricular fibrillation and can decrease infarct size, although it is still necessary to show whether this translates into an improvement in long-term morbidity and mortality. The benefit of chronic BBs in patients without ventricular dysfunction who have experienced an AMI is not established. Although they were commonly used in the past, the role of BBs in patients with hypertension without other comorbidities has lost prominence. Despite more than 4 decades of BB use, there are still clinical and experimental questions to be resolved, which makes this group of drugs one of the most fascinating at our disposal.
FUNDINGB. Ibáñez leads projects related to the subject of this review for the Spanish Society of Cardiology (2017 Translational Research Project) and the MICINN (Spanish Ministry of Science, Innovation, and Universities) through the Instituto de Salud Carlos III Health Research Fund (PI16/02110) and the European Regional Development Fund (ERDF: SAF2013-49663-EXP). The CNIC (National Center for Cardiovascular Research) is funded by the MICINN, the ISCiii, and the ProCNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
CONFLICTS OF INTERESTNone declared.