This real-world study—the first of its kind in a Spanish population—aimed to explore severe risk for cardiovascular events and all-cause death following exacerbations in a large cohort of patients with chronic obstructive pulmonary disease (COPD).
MethodsWe included individuals with a COPD diagnosis code between 2014 and 2018 from the BIG-PAC health care claims database. The primary outcome was a composite of a first severe cardiovascular event (acute coronary syndrome, heart failure decompensation, cerebral ischemia, arrhythmia) or all-cause death following inclusion in the cohort. Time-dependent Cox proportional hazards models estimated HRs for associations between exposed time periods (1-7, 8-14, 15-30, 31-180, 181-365, and >365 days) following an exacerbation of any severity, and following moderate or severe exacerbations separately (vs unexposed time before a first exacerbation following cohort inclusion).
ResultsDuring a median follow-up of 3.03 years, 18 901 of 24 393 patients (77.5%) experienced ≥ 1 moderate/severe exacerbation, and 8741 (35.8%) experienced the primary outcome. The risk of a severe cardiovascular event increased following moderate/severe COPD exacerbation onset vs the unexposed period, with rates being most increased during the first 1 to 7 days following exacerbation onset (HR, 10.10; 95%CI, 9.29-10.97) and remaining increased >365 days after exacerbation onset (HR, 1.65; 95%CI, 1.49-1.82).
ConclusionsThe risk of severe cardiovascular events or death increased following moderate/severe exacerbation onset, illustrating the need for proactive multidisciplinary care of patients with COPD to prevent exacerbations and address other cardiovascular risk factors.
Keywords
In Spain, chronic obstructive pulmonary disease (COPD) is a significant public health concern due to its estimated prevalence of nearly 12%1 and socioeconomic impact.2 In 2016, COPD was the fourth leading cause of mortality in Spain.3 Individuals living with COPD are at higher risk of cardiovascular disease vs those without COPD,4,5 due to shared risk factors and physiopathology.6,7 In Spain, the CONSISTE study found that the prevalence of cardiovascular disease was substantially higher in patients with vs without COPD: heart failure (HF, 25% vs 1%), arrhythmia (16% vs 5%), ischemic heart disease (13% vs 5%).8 These estimates were also found in the AvoidEx study in Spain regarding atrial fibrillation (AF, 15%), HF (14%), and ischemic heart disease (10%) in patients with COPD.9
Previous studies have reported increased cardiovascular event risk during and following COPD exacerbations,10–16 especially myocardial infarction (MI),11–13,15,16 stroke,11–16 HF,12 and arrhythmias.10 However, some findings are from post hoc analyses of randomized controlled trials,10,13 which may not reflect the real-world COPD population; others are from observational studies in the United Kingdom,11,16 United States,12 Denmark,15 and the Netherlands.14
The EXAcerbations of COPD and their OutcomeS on CardioVascular diseases (EXACOS-CV) is a program of separate retrospective observational longitudinal studies conducted in Canada, Spain, Italy, Germany, the Netherlands, the UK, Japan, the USA, France, and China, using the most appropriate administrative database in each country. The overall study design has been published.17 In publications from the EXACOS-CV program to date, consistent elevations in risk for cardiovascular events following exacerbations have been seen in patients with COPD in several countries, including Canada, Germany, the Netherlands, and the United Kingdom.18–21 Here, we report findings of the EXACOS-CV study in Spain. The objectives were to: a) estimate the incidence rate (IR) and risk of a severe cardiovascular event or all-cause death associated with time following a moderate/severe exacerbation, overall, and for individual types of cardiovascular events; b) estimate this association for moderate and severe exacerbations separately; and c) estimate this association in newly diagnosed patients.
METHODSStudy design and populationData were retrieved from the anonymized BIG-PAC database. BIG-PAC contains clinical data on approximately 2 million people from primary care centers and hospitals from 7 health care areas in 7 autonomous communities (1 area per community) in Spain. BIG-PAC data are derived from routinely collected medical information from primary health centers, referral hospitals, and pharmacies within the Spanish national health system. Information from different sources are integrated, anonymized, and transferred into the BIG-PAC database for research purposes. The database has been extensively used in recent years.22,23 Integrated medical records are available for general practitioner, specialist care, hospital, and emergency visits; outpatient and inpatient pharmacy dispensation; and date of death (registered by a primary care physician).
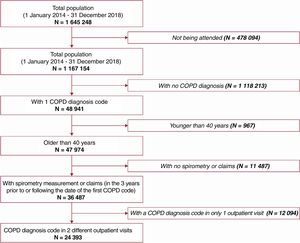
The cohort entry date was the first identified COPD diagnosis, which was ascertained in the database by either: ≥ 2 outpatient visits with COPD diagnosis codes, or ≥ 1 outpatient visit with a COPD diagnosis code and indication for spirometry measurements being performed (3 years before or following the COPD code date), or ≥ 1 inpatient COPD diagnosis code. If ≥ 2 diagnosis dates were identified through inpatient or outpatient visits, the earliest defined the cohort entry date. Eligible patients were aged ≥ 40 years at cohort entry with a COPD diagnosis between 1 January 2014 and 31 December 2018. This age inclusion criterion is considered a standard method for improving the specificity of an algorithm to detect individuals with COPD when using secondary data. Patients were excluded if they had alpha-1 antitrypsin deficiency (International Classification of Diseases, Ninth Revision [ICD-9 273.8 and 273.9] or Tenth Revision [ICD-10 E88.01]). Codes for COPD diagnosis and related exacerbations, as defined in the Spanish Ministry of Health coding manual, are listed in table 1 of the supplementary data.
The incident cohort was a subgroup of the overall cohort presumed to be newly diagnosed with COPD, as defined by having no COPD diagnosis code for 24 months before cohort entry.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Consorci Sanitari de Terrassa (protocol AZ-EXA-2022-06, 13 June 2022). This was a secondary data study using fully anonymized data and there was no requirement for patient consent.
Follow-upPatients were followed up from cohort entry until the outcome of interest or censoring (31 December 2019 or loss to follow-up), whichever came first.
ExposureThe exposures of interest were moderate/severe exacerbations occurring during follow-up. Moderate exacerbations were defined as those requiring an outpatient visit with a diagnosis code for COPD (table 1 of the supplementary data) and dispensation of oral corticosteroids and/or antibiotics within 5 days following the visit and for a maximum of 15 days. Records were checked to ensure oral corticosteroids and antibiotics were prescribed for exacerbation treatment. For antibiotics, it was also confirmed there was no concurrent acute nonpulmonary infection. Severe exacerbations were defined as those requiring an emergency department visit or a hospitalization of ≥ 1 night with a discharge diagnosis code for COPD exacerbation (table 1 of the supplementary data). Definitions of moderate and severe exacerbations used in this study were consistent with Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2022 definitions when the study was conducted.24
Exposed time was defined as the time following the onset of an exacerbation and was divided into 6 subperiods (1-7, 8-14, 15-30, 31-180, 181-365 days, and >365 days). Unexposed time was defined as time from cohort entry to the earliest of first moderate/severe exacerbation, or end of follow-up (occurrence of outcome or censoring) (figure 1 of the supplementary data).
OutcomesEvents of interest included: a) acute coronary syndrome (ACS; acute MI and unstable angina); b) HF decompensation; c) cerebral ischemia (ischemic stroke and transient ischemic attack); d) arrhythmias (newly diagnosed AF and other arrhythmias, including resuscitated cardiac arrest), and e) all-cause death. Nonfatal severe cardiovascular events were those resulting in hospitalization for ≥ 1 night with a main or secondary discharge code for the event not leading to death in the hospital (table 2 of the supplementary data). Deaths were identified through primary care physician records, with in-hospital cause of death determined by admission diagnosis codes and categorized into “respiratory”, “cardiac”, or “other”; deaths outside the hospital were categorized as “unknown”.
The primary endpoint was the composite of time to a first severe nonfatal cardiovascular event of any type or all-cause death. Secondary endpoints included time to a first severe nonfatal cardiovascular event of each type, with death as a censoring event.
Statistical analysisAnalyses were performed using SPSSWIN version 23 and R Project for Statistical Computing, version 4.0.3. Missing data were not imputed. Medical code absence was considered absence of the condition.
Analyses were conducted in the overall cohort (ie, all eligible patients) and incident cohort. Demographics and baseline characteristics in both cohorts are described in full and in patients who did or did not have ≥ 1 exacerbation during follow-up. A covariate balance plot was used to compare baseline characteristics between patients who ever and never had a moderate or severe exacerbation during follow-up (figure 2 of the supplementary data).
Crude IRs per 100 patient-years with 95% exact Poisson confidence intervals (95%CIs) were calculated for all primary and secondary outcome events of interest within unexposed and exposed time periods following moderate/severe exacerbation onset.
Cox proportional hazards regression models25 were fitted with binary indicators of each exposed time period as time-dependent covariates to estimate hazard ratios (HR) for changes in rates of: a) the primary endpoint following a moderate/severe exacerbation; b) the primary endpoint following a moderate or a severe exacerbation separately; c) each secondary and other endpoint, following a moderate/severe exacerbation; d) the primary endpoint following a first, second, and third (or more) moderate/severe exacerbation in the incident cohort.
Models were fitted with and without adjustment for prespecified confounders. These included age, sex, cohort entry year, smoking status, alcohol use disorder, comorbidities, primary care general practitioner visits, number of prior exacerbations, and medication use, along with cardiovascular risk factors such as diabetes, hypertensive disease, and prior HF, among others (described in table 1). Analyses of individual cardiovascular event types and all-cause death included competing cardiovascular events as time-dependent covariates. For nonfatal cardiovascular events, patients were right censored at time of death.
Demographics and baseline characteristics in patients with COPD overall, and categorized as having at least one or no exacerbations during follow-up
| All patients | No exacerbation during total follow-upa | ≥ 1 exacerbation during total follow-upa | |
|---|---|---|---|
| N=24 393 | N=5492 | N=18 901 | |
| Age, y | |||
| Mean | 67.9±11.6 | 62.4±4.3 | 69.5±12.5 |
| Median | 68.2 [59.2–76.5] | 63.5 [59.8–65.9] | 71.8 [58.7–78.7] |
| Sex (female)b | 5308 (21.8) | 1194 (21.7) | 4114 (21.8) |
| Behavioral risk factors | |||
| Tobacco use | |||
| Smoker | 7800 (32) | 3725 (67.8) | 4075 (21.6) |
| Ex-smoker | 12 302 (50.4) | 801 (14.6) | 11 501 (60.8) |
| Never smoker | 4291 (17.6) | 966 (17.6) | 3325 (17.6) |
| Alcohol use disorder | 1044 (4.3) | 356 (6.5) | 688 (3.6) |
| Associated comorbidities | |||
| Obesity | 4131 (16.9) | 1349 (24.6) | 2782 (14.7) |
| Diabetes mellitus type 2 | 4190 (17.2) | 611 (11.1) | 3579 (18.9) |
| Disorders of lipoprotein metabolism | 12 263 (50.3) | 3972 (72.3) | 8291 (43.9) |
| Ischemic heart diseases | 4345 (17.8) | 686 (12.5) | 3659 (19.4) |
| Hypertensive diseases | 14 576 (59.8) | 2834 (51.6) | 11 742 (62.1) |
| HF and pulmonary edema | 5311 (21.8) | 829 (15.1) | 4482 (23.7) |
| Pulmonary hypertension | 833 (3.4) | 113 (2.1) | 720 (3.8) |
| Venous thromboembolism | 1833 (7.5) | 257 (4.7) | 1576 (8.3) |
| Cerebrovascular disease | 2408 (9.9) | 325 (5.9) | 2083 (11) |
| Arrhythmia | 2765 (11.3) | 409 (7.4) | 2356 (12.5) |
| Current asthma | 2490 (10.2) | 373 (6.8) | 2117 (11.2) |
| Chronic kidney disease, renal failure | 2598 (10.7) | 350 (6.4) | 2248 (11.9) |
| Severe mental illness | 3633 (14.9) | 513 (9.3) | 3120 (16.5) |
| Anxiety disorder | 9248 (37.9) | 1745 (31.8) | 7503 (39.7) |
| Number of GP visits in the 12 months preceding cohort entry | |||
| Mean | 7±3.5 | 3.9±2.2 | 7.9±3.3 |
| Median | 7 [5–9] | 4 [2–5] | 8 [6–10] |
| Number of exacerbations (moderate/severe) in the 12 months preceding cohort entry | |||
| 0 | 20 153 (82.6) | 5074 (92.4) | 15 079 (79.8) |
| 1 | 2139 (8.8) | 382 (7.0) | 1757 (9.3) |
| 2 | 1312 (5.4) | 36 (0.7) | 1276 (6.8) |
| ≥ 3 | 789 (3.2) | 0 (0) | 789 (4.2) |
| Medication usec | |||
| Long-acting inhaled COPD drug use | 22 433 (92) | 4948 (90.1) | 17 485 (92.5) |
| ICS | 14 451 (59.2) | 3063 (55.8) | 11 388 (60.3) |
| LABA | 16 127 (66.1) | 3409 (62.1) | 12 718 (67.3) |
| LAMA | 10 617 (43.5) | 2248 (40.9) | 8369 (44.3) |
| Short-acting inhaler | 23 432 (96.1) | 5000 (91.0) | 18 432 (97.5) |
| SABA | 23 307 (95.5) | 4945 (90.0) | 18 362 (97.1) |
| SAMA | 2546 (10.4) | 527 (9.6) | 2019 (10.7) |
| Roflumilast | 447 (1.8) | 83 (1.5) | 364 (1.9) |
| Any cardiac drug | 22 285 (91.4) | 4912 (89.4) | 17 373 (91.9) |
| Any metabolic drug | 15 188 (62.3) | 3248 (59.1) | 11 940 (63.2) |
COPD, chronic obstructive pulmonary disease; GP, general practitioner; HF, heart failure; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist.
Data are expressed as No. (%), mean ± standard deviation or median [interquartile range].
Of 1 645 248 individuals in the BIG-PAC database, 24 393 were included in the overall cohort (figure 1) among whom 12 713 were newly diagnosed with COPD (incident cohort). Demographic and baseline characteristics are presented in the overall cohort (table 1) and incident cohort (table 3 of the supplementary data). Between patients who ever and never had a moderate/severe exacerbation during follow-up, previous exacerbations and age were the covariates that differed most between groups, with standardized mean differences exceeding 0.1 (figure 2 of the supplementary data). The median [interquartile range] follow-up until a first cardiovascular event, death or censoring was 3.03 [1.04–5.90] and 2.46 [1.20–3.99] years in the overall and incident cohorts, respectively. In the overall cohort: 14 299/24 393 (58.6%) patients experienced ≥ 1 moderate exacerbation and 2314 (9.5%) experienced ≥ 1 severe exacerbation before a first cardiovascular event or censoring, and 18 865/24 393 (77.3%) patients experienced ≥ 1 moderate exacerbation and 5270/24 393 (21.6%) patients experienced ≥ 1 severe exacerbation during total follow-up. Overall, 18 901/24 393 (77.5%) patients experienced ≥ 1 moderate/severe exacerbation, with a mean ± standard deviation of 3.9±2.9 exacerbations per patient during total follow-up.
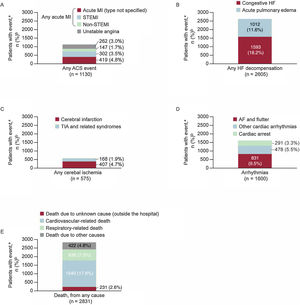
Overall cohort analysesSevere cardiovascular events, including all-cause death, following exacerbation onsetDuring follow-up, 8741/24 393 of patients from the overall population (35.8%) experienced ≥ 1 severe nonfatal cardiovascular event (n=5910; any ACS, HF decompensation, cerebral ischemia, or arrhythmia) or all-cause death (n=2831; figure 2). The most common nonfatal severe cardiovascular event (29.8%) was any HF decompensation (2605/8741), and out of 2831 deaths by any cause, cardiovascular-related deaths were the most common (1540; 54.4%).
First event of interest (nonfatal severe cardiovascular events, by type or all-cause death) during follow-up. a In patients with multiple cardiovascular events during follow-up, only the first one is listed, such that the sum of patients in each category equals the total number of patients. b Percentage calculated using the total number of patients with events (8741) as the denominator. ACS, acute coronary syndrome; AF, atrial fibrillation; HF, heart failure; MI, myocardial infarction; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack.
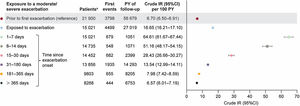
Crude IR (95%CI) per 100 patient-years of a first severe cardiovascular event of any type, or all-cause death, was 6.70 (6.50-6.91) during unexposed time before an exacerbation (or censoring in the absence thereof). Crude IRs increased 1 to 7 days following moderate/severe exacerbation onset (64.61 [61.67-67.44]) and decreased to 7.98 (7.42-8.59) at 181 to 365 days and 6.57 (6.01-7.19) >365 days after exacerbation onset (figure 3). Table 4 of the supplementary data presents crude IRs by cardiovascular event type.
Crude IRs (95%CI) of a first severe cardiovascular event (nonfatal event or all-cause death) within unexposed and exposed time periods following moderate/severe exacerbation onset. Shaded time periods (including time prior to first exacerbation and all follow-up time of never exacerbating patients) were considered unexposed to exacerbations. a Number of patients contributing at least 1 day of data in this period from the overall population of 24 393 patients. 95%CI, 95% confidence interval; IR, incidence rate; PY, patient-years.
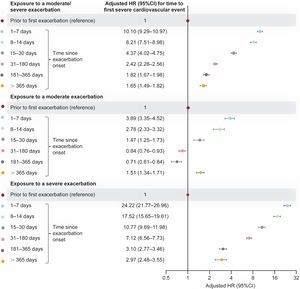
Relative to unexposed time, the rate of a first severe cardiovascular event, including all-cause death, increased 10.1-fold at 1 to 7 days following moderate/severe exacerbation onset; the rate remained increased >365 days after exacerbation onset (fully adjusted HRs, figure 4; unadjusted HRs, table 5 of the supplementary data).
Adjusted HR (95%CI) for time to a first severe cardiovascular event (nonfatal event or all-cause death) within exposed time periods following moderate/severe, moderate, and severe exacerbation onset, vs unexposed time (fully adjusted multivariable model). Shaded time periods (including time prior to first exacerbation and all follow-up time of never exacerbating patients) were considered unexposed to exacerbations. 95%CI, 95% confidence interval; HR, hazard ratio.
Similar patterns of increased risk were observed for periods following onset of moderate exacerbations and periods following onset of severe exacerbations (figure 4). There were 24.2-fold and 17.5-fold rate increases 1 to 7 and 8 to 14 days after severe exacerbation onset, respectively. Risk following a severe exacerbation decreased over time, but remained significantly higher at >365 days after exacerbation onset vs unexposed time. There were 3.9-fold and 2.8-fold rate increases 1 to 7 and 8 to 14 days since moderate exacerbation onset, respectively.
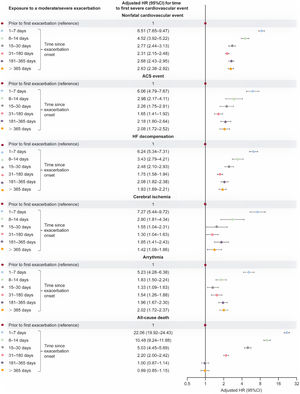
Severe cardiovascular event risk, including all-cause death, by event type, following exacerbation onsetRisk of each nonfatal cardiovascular event type was increased during each specified period in the 365 days following moderate/severe exacerbation onset, with substantial increases observed in the first 2 weeks (fully adjusted HRs, figure 5; unadjusted HRs, table 5 of the supplementary data). Rates of all-cause death increased 22.1-fold and 10.5-fold at 1 to 7 and 8 to 14 days after exacerbation onset, respectively, and remained elevated 31 to 180 days after exacerbation onset.
Adjusted HR (95%CI) for time to a first severe cardiovascular event (by event type) within exposed time periods following moderate/severe exacerbation onset vs unexposed time (fully adjusted multivariable model). Shaded time periods (including time prior to first exacerbation and all follow-up time of never exacerbating patients) were considered unexposed to exacerbations. ACS, acute coronary syndrome; 95%CI, 95% confidence interval; HF, heart failure; HR, hazard ratio.
During follow-up for the primary outcome, 7216/12 713 (56.8%) incident COPD patients had ≥ 1 moderate/severe exacerbation.
During the total study follow-up period, 9225/12 713 (72.6%) incident patients had ≥ 1 moderate/severe exacerbation, 8551/12 713 (67.3%) had ≥ 2 moderate/severe exacerbations, and 6652/12 713 (52.3%) had ≥ 3 moderate/severe exacerbations.
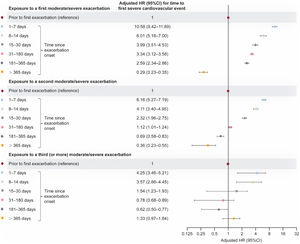
The largest increase in risk of a first severe cardiovascular event occurred after the first moderate/severe exacerbation onset; exposure to a second and third (or more) exacerbation also increased cardiovascular event risk vs unexposed time before the first exacerbation (figure 6). IR were greatest 1 to 7 days after exacerbation onset and tended to be lower during periods further from exacerbation onset.
Adjusted HR (95%CI) for time to a first severe cardiovascular event of any type (nonfatal event or all-cause death) within exposed time periods following onset of a first, second, and third (or more) moderate/severe exacerbation vs unexposed time (fully adjusted multivariable model; newly diagnosed patients). Shaded time periods (including time prior to first exacerbation and all follow-up time of never exacerbating patients) were considered unexposed to exacerbations. HRs quantify the separate risks of a severe cardiovascular event following a first, second, and third (or more) moderate/severe exacerbation vs unexposed time without an exacerbation. 95%CI, 95% confidence interval; HR, hazard ratio.
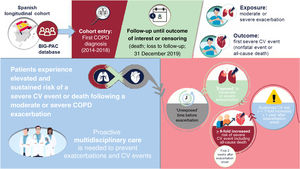
In this real-world study, evaluating cardiovascular event risk following COPD exacerbations in Spain, exacerbations were associated with an increased risk of severe cardiovascular event or all-cause death. Risk substantially increased in the year following a moderate/severe exacerbation, with the greatest increases observed during the first 2 weeks following exacerbation onset. An overview of the study, results, and interpretation is provided in the figure 7.
Cardiovascular risk increased following both moderate and severe exacerbations. While severe exacerbations increased risk to a greater extent than moderate exacerbations, and did so for longer (ie, beyond 1 year), severe cardiovascular event risk was still substantially increased up to 30 days following moderate exacerbation onset. Despite the strict definition used for moderate exacerbations, 77.3% of patients experienced ≥ 1 moderate exacerbation during total follow-up, which averaged approximately 3 years. The high moderate exacerbation rate in this Spanish population, and its association with severe cardiovascular events, emphasizes the importance of preventing any exacerbation regardless of severity.
Increased risk was seen among different cardiovascular event types, indicating that a range of pathophysiological mechanisms may be involved. The enduring severe cardiovascular event risk for up to 1 year after an exacerbation suggests the involvement of long-term mechanisms beyond the systemic inflammation associated with the acute exacerbation period. Various mechanisms have been suggested to explain how COPD elevates risk of cardiac stress and cardiopulmonary events, including systemic inflammation,7,26 hyperinflation,7,27 hypoxia,7 and endothelial dysfunction.28,29 These mechanisms may be augmented during exacerbations, with the additional effect of hypercoagulability.7,26–28 Even incident patients who experienced a first moderate/severe exacerbation had an increased risk of severe cardiovascular events. These findings further highlight the importance of preventing all exacerbations and should prompt future research to elucidate how cardiovascular risk following exacerbations may depend on the cumulative number of exacerbations.
Regarding nonfatal cardiovascular events, adjusted HRs for ACS and HF decompensation were among the most common in the first week following exacerbation onset and >365 days after an exacerbation. Additionally, the most common first nonfatal cardiovascular event was HF decompensation. This is clinically important for the Spanish population because ACS is a leading cause of death and morbidity,30 and HF is a major public health concern;31,32 both ACS and HF are associated with substantial health care costs.30,31 Notably, hospital admissions for HF increased from 2003 through 2015,32 and the HF-PATHWAYS study indicated a need to improve treatment optimization in patients with HF with reduced ejection fraction, which was a phenotype reported in 51.7% of patients in that analysis.22
In this analysis, lower cardiovascular event risk vs unexposed time was observed 31 to 365 days after a moderate exacerbation (HRs <1) vs higher risk >365 days postexacerbation (HR >1). The reason for this is unclear, but it could be related to unmeasured confounding. A depletion of susceptible cases could also contribute to the findings >365 days postexacerbation.
The overall findings from this population of patients with COPD from Spain are consistent with observations from other countries included in the EXACOS-CV program.18–21 As in the current study, in EXACOS-CV studies from the United Kingdom,18 Canada,19 the Netherlands20 and Germany,21 in the 12 months following the onset of a moderate/severe exacerbation, cardiovascular event rates were highest in the first 2 weeks postexacerbation. Among countries, cardiovascular event risk peaked 1 to 14 days postexacerbation in the United Kingdom (adjusted HR, 3.19), and 1 to 7 days postexacerbation in Canada (adjusted HR, 15.86), the Netherlands (adjusted HR, 15.3), and Germany (adjusted HR, 8.6). In the current analysis, cardiovascular event risk in Spain also peaked at 1 to 7 days postexacerbation (adjusted HR, 10.10), indicating that these data are roughly in line with the range of values observed in other countries. Additionally, though HRs for increased cardiovascular risk decreased gradually further from exacerbation onset, elevated risk persisted for up to 1 year among all countries (adjusted HR range, 1.08-1.84).
This study also provides insight into the cardiovascular burden in Spanish patients with COPD. In the overall cohort at baseline, the prevalence of cardiovascular and metabolic-related disease, including obesity, diabetes mellitus type 2, lipoprotein metabolism disorders, ischemic heart disease, hypertensive diseases, HF, and arrhythmia was >10%. This finding is in agreement with previous studies of cardiovascular risk factors in patients with COPD in Spain.5,8 In the Spanish CONSISTE study, the prevalence of HF (24.7% vs 1.4%), arrhythmia (16.1% vs 4.7%), and ischemic heart disease (12.5% vs 4.7%) was significantly higher in patients with vs without COPD.8 The presence of these conditions in the current study resulted in most patients using cardiac (91.4%) or metabolic (62.3%) medications. Furthermore, during unexposed time, there was substantial incidence of cardiovascular events, with an IR (95%CI) per 100 patient-years of 6.70 (6.50-6.91) for a first severe cardiovascular event. For comparison, in a population-based cohort study in Catalonia in 2018, the IRs of cardiovascular disease per 1000 patient-years ranged between 6.64 and 22.8 (men) and 2.58 and 16.32 (women), among those aged between 40 and ≥ 70 years.33 Therefore, even in the absence of exacerbations, Spanish patients with COPD have a high prevalence of cardiovascular-related comorbidities.
This high prevalence of cardiovascular and metabolic morbidities, together with the observed link between COPD exacerbations and severe cardiovascular events, highlights the substantial burden of cardiopulmonary risk in this population. Spanish COPD guidelines (GesEPOC) recommend considering whether cardiovascular disease is present when a patient's COPD is poorly controlled, despite receiving inhaled treatment and their primary clinical expression is dyspnea.34 Strategies to reduce cardiopulmonary risk and premature mortality should include optimization of cardiovascular treatments to manage cardiovascular risk, and COPD treatments to prevent exacerbations. A previous study in Spain, which also used the BIG-PAC database, supports the importance of treatment optimization, reporting that patients with COPD initiating single inhaler triple therapy experience clinically meaningful improvements in treatment persistence vs multiple inhaler triple therapy, with resulting lower exacerbation rates and mortality.23 Other studies have also demonstrated prompt initiation of therapy reduces exacerbation risk and improves outcomes.35,36
While this study demonstrated that COPD exacerbations are independent risk factors for a severe cardiovascular event, causality cannot be easily established. However, aspects of the study and external sources of information may act as criteria for causality as suggested by Hill.37 First, other studies exploring the association between COPD exacerbations and subsequent cardiovascular events consistently provide similar findings, irrespective of study design and database used,10,13,18–21 and the strength of the association found in the current study is substantial. Second, exposure preceded the outcome, as suggested in periods of time beyond the symptomatic phase (1-7 days). Third, there are well-described pathophysiological mechanisms underpinning the association between COPD and the increased risk of cardiovascular anatomical or functional impairment,6,7 suggesting that an exacerbation of COPD can act as a trigger for severe cardiovascular events.
LimitationsThe limitations of this study should be considered when interpreting these data, including variable data quality from electronic health records, particularly regarding the risk of diagnosis errors. For example, overlapping symptoms between COPD and HF can hamper differential diagnosis.38,39 There is commonality in the experience of dyspnea among patients with COPD and those with congestive HF.40 As there is risk of misdiagnosing a severe acute cardiovascular event as a COPD exacerbation, the 1 to 7 day period following exacerbation onset could be prone to misclassification and should be interpreted accordingly. However, elevated risk beyond the initial 7-day period and then up to 365 days and >365 days, suggests these findings are not due solely to misclassification. Additionally, there is a risk of missing an exacerbation or cardiovascular event when using a secondary database, especially if the patient did not inform their physician or attend a hospital that collected data used in the study. In this study, medical code absence was considered the absence of the condition; in claims databases a code (ie, for a diagnosis or procedure), is either present, meaning the patient has the diagnosis/procedure, or not present, meaning that the patient either does not have the disease/procedure or the information is missing. Consequently, truly missing information related to medical claims cannot be identified or imputed. In the current analyses, all patients contributed to the models.
Unmeasured confounding and cohort effects could also impact the results. In particular, a survivor effect on risk could be associated with a second and third (or more) exacerbation, as patients need to stay in the cohort long enough to experience these events. Last, while biomarkers of congestion (eg, B-type natriuretic peptide) or myocardial injury (eg, high-sensitivity troponins) were not included here, their inclusion could be valuable for informing an early differential diagnosis and initiating appropriate treatment.
A key strength of this study is that it used a population-based cohort of patients with COPD, without selecting only those with the most severe disease. Additionally, the BIG-PAC database is representative of the Spanish population,22 and consequently these findings are generalizable to the real-world population with COPD in Spain.
CONCLUSIONSThis study explored the risk of severe cardiovascular events and death for the first time in a large cohort of patients with COPD in Spain. The prevalence of cardiovascular disease was high, and rates of hospitalization for a severe cardiovascular event or all-cause death were elevated for ≥ 180 days after onset of an exacerbation of COPD. While severe exacerbations were associated with increased cardiovascular event risk, even 1 moderate exacerbation also increased risk; therefore the clinical impact of moderate exacerbations should not be minimized.
These findings emphasize the need for the proactive multidisciplinary care of patients with COPD to prevent exacerbations and potentially address other cardiovascular risk factors and comorbidities. Proactive management should include improving goals for the prevention of cardiopulmonary events in patients with COPD and optimizing pharmacotherapies for cardiovascular disorders and COPD.
- -
Substantial cardiovascular burden exists in Spanish patients with COPD.
- -
Both moderate and severe COPD exacerbations increase the risk of severe cardiovascular events and death.
- -
No previous real-world study has assessed the risk of cardiovascular events following moderate and severe COPD exacerbations in Spain.
- -
In this analysis, the greatest IR increases occurred ≤ 2 weeks after exacerbation onset.
- -
Increased cardiovascular event risk was sustained for at least 1 year after exacerbation onset.
- -
Findings from this study emphasize the need for the proactive multidisciplinary care of patients to reduce cardiopulmonary risk and premature mortality.
This study was funded by AstraZeneca.
ETHICAL CONSIDERATIONSThe study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Consorci Sanitari de Terrassa (protocol AZ-EXA-2022-06, 13 June 2022). This was a secondary data study using fully anonymized data and patient consent was not required. Sex was considered as a confounder in our models (as described in the final paragraph of the methods) and sex dimensions are provided in the results. Sex was reported in the electronic health records retrieved from the anonymized BIG-PAC database, containing data from hospitals and primary care centres.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence software was not used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONSConceptualization, S. Santos, J. Sánchez-Covisa, K. Rhodes and C. Nordon; project administration, J. Sánchez-Covisa, I. Hernández, C. Corregidor and C. Nordon; resources, J. Sánchez-Covisa, I. Hernández, C. Corregidor and C. Nordon; methodology, J. Sánchez-Covisa, L. Escudero, K. Rhodes and C. Nordon; data curation, I. Hernández; formal analysis, I. Hernández; validation, S. Santos, N. Manito, J. Sánchez-Covisa, I. Hernández, C. Corregidor, L. Escudero and C. Nordon; investigation, J. Sánchez-Covisa, C. Corregidor, K. Rhodes and C. Nordon; visualization, S. Santos, N. Manito, J. Sánchez-Covisa, I. Hernández, C. Corregidor, L. Escudero and C. Nordon; supervision, S. Santos, N. Manito, J. Sánchez-Covisa, I. Hernández, C. Corregidor, L. Escudero, K. Rhodes and C. Nordon; funding, J. Sánchez-Covisa, L. Escudero, C. Nordon; writing—reviewing and editing, S. Santos, N. Manito, J. Sánchez-Covisa, I. Hernández, C. Corregidor, L. Escudero, K. Rhodes and C. Nordon.
CONFLICTS OF INTERESTS. Santos reports presentations and/or scientific advisory board fees from AstraZeneca, Chiesi, GlaxoSmithKline, Grifols, Menarini, Novartis and Pfizer. N. Manito reports medical lecture and advisory board fees from AstraZeneca. J. Sánchez-Covisa, C. Corregidor, L. Escudero, K. Rhodes and C. Nordon are employees of AstraZeneca and may hold stock and/or stock options in the company. I. Hernández is an employee of Atrys Health, which was contracted by AstraZeneca to conduct this study.
Medical writing support, under the guidance of the authors, was provided by Sarah Piggott, MChem, and Kirsty Ainscough, PhD, CMC Connect, a division of IPG Health Medical Communications, and was funded by AstraZeneca, in accordance with Good Publication Practice (GPP 2022) guidelines.41