Ionizing radiation exposure in catheter ablation procedures carries health risks, especially in pediatric patients. Our aim was to compare the safety and efficacy of catheter ablation guided by a nonfluoroscopic intracardiac navigation system (NFINS) with those of an exclusively fluoroscopy-guided approach in pediatric patients.
MethodsWe analyzed catheter ablation results in pediatric patients with high-risk accessory pathways or supraventricular tachycardia referred to our center during a 6-year period. We compared fluoroscopy-guided procedures (group A) with NFINS guided procedures (group B).
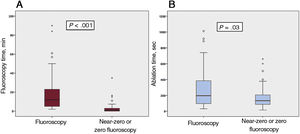
ResultsWe analyzed 120 catheter ablation procedures in 110 pediatric patients (11±3.2 years, 70% male); there were 62 procedures in group A and 58 in group B. We found no significant differences between the 2 groups in procedure success (95% group A vs 93.5% group B; P=.53), complications (1.7% vs 1.6%; P=.23), or recurrences (7.3% vs 6.9%; P = .61). However, fluoroscopy time (median 1.1minutes vs 12minutes; P <.0005) and ablation time (median 96.5seconds vs 133.5seconds; P=.03) were lower in group B. The presence of structural heart disease was independently associated with recurrence (P=.03).
ConclusionsThe use of NFINS to guide catheter ablation procedures in pediatric patients reduces radiation exposure time. Its widespread use in pediatric ablations could decrease the risk of ionizing radiation.
Keywords
In recent years, catheter ablation has become established as the treatment of choice for supraventricular tachycardia (SVT) in the pediatric population.1,2 Retrospective and prospective studies have shown it to be a safe and effective method even in infants younger than 1 year.3–7
Fluoroscopy with ionizing radiation has been used to guide ablation procedures since their inception; however, it has harmful effects for both the practitioner8 and the patient, and the pediatric population is particularly sensitive to these effects.10–12 Given that there is a definite risk of cancer induction9 and no minimum safe threshold dose, the radiation dose should be reduced to the lowest possible according to the ALARA principle (ie, As Low As Reasonably Achievable).
Nonfluoroscopic intracardiac navigation systems (NFINSs) have been shown to be effective in ablation procedures with near zero or zero fluoroscopy.13 However, their use is uncommon in Spain even though the pediatric population is more vulnerable to radiation damage and previous studies have shown the safety and efficacy of SVT treatment with near zero or zero fluoroscopy.14–16 A recent questionnaire-style survey of electrophysiologists from 42 European centers showed that NFINSs were used in only 23% of cases of SVT.17 The recent results of the longest series of pediatric ablations recorded in Spain showed that a NFINS was used in only 20% of procedures.18
Our aim was to compare the safety and efficacy of NFINS-guided catheter ablation with those of a standard fluoroscopy-guided approach in a consecutive series of pediatric patients with high-risk accessory pathways (AP) or SVT treated with ablation.
METHODSSingle-center retrospective observational study conducted over a 6-year period (January 2013 to January 2019) that included all consecutive patients younger than 16 years undergoing ablation at a regional referral center. The protocol was approved by the Ethics Committee of our hospital.
Patients and study groupsA NFINS was used to guide diagnostic catheter placement, mapping, and ablation. It was used with increasing frequency as the learning curve progressed, rising from 20% in 2013 to 100% from 2017 onward. The main reason for using a NFINS was to minimize fluoroscopy (near zero fluoroscopy approach) or eliminate it altogether (zero fluoroscopy approach).
All procedures were performed by the same operator with extensive experience in pediatric ablation.
We recorded the sociodemographic characteristics of the patients, weight, presence or absence of heart disease, substrate treated, NFINS chosen, and procedure times (ie, from the beginning of puncture to catheter removal), fluoroscopy times, and ablation times (ie, calculated as the sum in seconds of each radiofrequency or cryoenergy until success or termination of the procedure due to failure). We also recorded the energy source used, procedural success, complications, and recurrences in the short (6 months) and long-term (until the end of follow-up). We compared fluoroscopy-guided procedures (group A) with NFINS-guided procedures (group B).
Electrophysiological studyAll procedures were performed under general anesthesia. The left brachial vein was cannulated to introduce the coronary sinus catheter and the femoral vein for the other catheters, except when the target substrate was a right AP in the right superior paraseptal region, in which case the jugular access route was used as the first option.19
Two decapolar diagnostic catheters were used, one for the coronary sinus and the other positioned in the right ventricular apex for simultaneous recording of the hisiogram. The NFINS used was the CARTO3 system (Biosense Webster, United States).
A nonfluoroscopic procedure was planned when the electrocardiogram (ECG) indicated an arrhythmic substrate in the right cavities (intranodal tachycardia [INT], right atrial tachycardia [AT], or presence of AP with probable right location). The ablation catheter has a magnetic sensor and is visualized along the entire course of the vascular system. It creates an electrical matrix on which the sensorless catheters are visualized once they reach this matrix. The ablation catheter created a 3-dimensional reconstruction of the right atrium, venae cavae, and coronary sinus that facilitated placement of the sensorless catheters.
A near zero fluoroscopy procedure was planned in patients with asymptomatic preexcitation or clinical palpitations without documented tachycardia, in which ablation might not be required, to avoid the unnecessary use of an ablation catheter. All left APs were approached via the retroaortic route and fluoroscopy was used to traverse the aortic valve. A 30-minute waiting time after successful application was established for all procedures.
AblationThe arrhythmic substrate was classified as atrioventricular AP, INT, or AT. A nonirrigated catheter with a 4-mm tip was used as the radiofrequency ablation catheter, and radiofrequency energy was applied at 20W to 40W. Cryoablation was performed using a 6-mm catheter with cryomapping between -30C and -50C and cryoablation at -70C. Because the cryoablation catheter is not fitted with a magnetic sensor, the procedure initially required the use of a radiofrequency ablation catheter with a sensor to create the electroanatomic reconstruction, mark the point with greater precocity, and record the catheter shadow as a reference. It was then exchanged for the cryoablation catheter, which had been configured as a diagnostic catheter in the navigator so that it could be displayed on the map that had already been created.
Standard criteria were used to determine acute procedural success, which was defined as the suppression or modulation of the slow pathway in the case of INT (the presence of up to 1 nodular echo was accepted), as the absence of inducibility in the case of AT, and as the absence of bidirectional conduction after adenosine triphosphate infusion in the case of AP.20,21
Follow-upPatients were discharged the day after ablation following an ECG to rule out complications or early recurrence in the case of Wolff-Parkinson-White syndrome.
Because our hospital is a referral center, follow-up visits were made to the hospital of origin: nevertheless, in all cases, 1 visit per year was made to the pediatric cardiology service of our hospital. Recurrence was defined as symptomatic recurrence with electrocardiographic documentation of tachyarrhythmia or recurrence of ventricular preexcitation.
We recorded early recurrences as those that occurred within 6 months after ablation and late recurrences as those that occurred between 6 months after ablation and the end of follow-up.
Statistical analysisQualitative variables were compared using the chi-square test or Fisher exact test and quantitative variables were compared using the Student t test if they followed a normal distribution or the Mann-Whitney U test otherwise. A P value of <.05 was used as a cutoff for statistical significance. We established predictors of recurrence by selecting variables related to a recurrence episode in the first 6 months after ablation (P <.1) and comparing them using multivariable logistic regression. We included all significant variables in the multivariate analysis and used a stepwise method to select the model that only included significant variables. The analysis included the use of NFINSs because it was the main variable in the study. All analyses were conducted using SPSS v. 21.0 (United States).
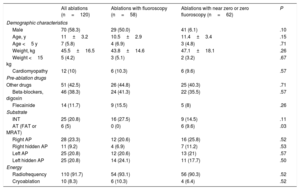
RESULTSBaseline characteristicsBetween January 2013 and April 2019, we performed 120 ablation procedures in 110 pediatric patients (70% male) with high-risk AP or SVT. Mean age was 11±3.2 (range, 0.1-15.9) years; mean weight was 45.5±16.5kg (2.5-83kg). In total, 5.8% of the children were younger than 5 years and 4.2% weighed less than 15kg. Seven of the procedures were redo procedures. Structural heart disease was present in 10% of the children: bicuspid aortic valve disease (n=2), mitral prolapse (n=2), Ebstein anomaly (n=2), noncompaction cardiomyopathy, hypertrophic cardiomyopathy (n=2), corrected single ventricle, and surgically closed subaortic ventricular septal defect (n=2). A total of 42.5% had received an antiarrhythmic drug: of these, the most frequently used were beta-blockers (38%).
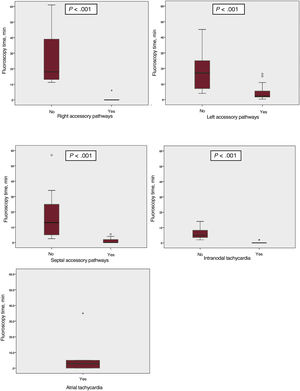
The most frequently ablated substrates were AP (74.2%), followed by INT (20.8%), and AT (5%). Regarding AP location, 50 (56.2%) were left APs and 23 (25.8%) were septal APs (12 [10%] right inferior paraseptal, 4 [3.3%] right inferior paraseptal decremental, 2 [1.7%] midseptal, and 5 [4.2%] right superior paraseptal). There were 16 (18%) right APs, one of which was a Mahaim AP. Table 1 shows the different substrates treated.
Baseline characteristics, substrates treated, ablation energy applied, and location of accessory pathways in both groups
| All ablations (n=120) | Ablations with fluoroscopy (n=58) | Ablations with near zero or zero fluoroscopy (n=62) | P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Male | 70 (58.3) | 29 (50.0) | 41 (6.1) | .10 |
| Age, y | 11±3.2 | 10.5±2.9 | 11.4±3.4 | .15 |
| Age <5 y | 7 (5.8) | 4 (6.9) | 3 (4.8) | .71 |
| Weight, kg | 45.5±16.5 | 43.8±14.6 | 47.1±18.1 | .26 |
| Weight <15 kg | 5 (4.2) | 3 (5.1) | 2 (3.2) | .67 |
| Cardiomyopathy | 12 (10) | 6 (10.3) | 6 (9.6) | .57 |
| Pre-ablation drugs | ||||
| Other drugs | 51 (42.5) | 26 (44.8) | 25 (40.3) | .71 |
| Beta-blockers, digoxin | 46 (38.3) | 24 (41.3) | 22 (35.5) | .57 |
| Flecainide | 14 (11.7) | 9 (15.5) | 5 (8) | .26 |
| Substrate | ||||
| INT | 25 (20.8) | 16 (27.5) | 9 (14.5) | .11 |
| AT (FAT or MRAT) | 6 (5) | 0 (0) | 6 (9.6) | .03 |
| Right AP | 28 (23.3) | 12 (20.6) | 16 (25.8) | .52 |
| Right hidden AP | 11 (9.2) | 4 (6.9) | 7 (11.2) | .53 |
| Left AP | 25 (20.8) | 12 (20.6) | 13 (21) | .57 |
| Left hidden AP | 25 (20.8) | 14 (24.1) | 11 (17.7) | .50 |
| Energy | ||||
| Radiofrequency | 110 (91.7) | 54 (93.1) | 56 (90.3) | .52 |
| Cryoablation | 10 (8.3) | 6 (10.3) | 4 (6.4) | .52 |
AP, accessory pathway; AT, atrial tachycardia; FAT, focal atrial tachycardia; INT, intranodal tachycardia; MRAT, macroreeentrant atrial tachycardia.
Data are expressed as No. (%) and mean±standard deviation.
Most of the ablation procedures were performed with radiofrequency energy (91.7%). Cryoablation was performed in 10 procedures: 5 INT, 3 right superior paraseptal APs, 1 midseptal AP, and 1 right inferior paraseptal decremental AP.
An exclusively fluoroscopy-guided approach was used in 58 patients (group A) and a NFINS was used in 62 (group B). In group B, near zero fluoroscopy was used in 31 patients and zero fluoroscopy in 31. However, fluoroscopy had to be used in 4 patients, thus reducing the final number to 27 patients.
Baseline characteristics, preablation medications, and radiofrequency energy applied were similar in the 2 groups (table 1). Cryoablation was used in 6 patients (10.9%) in group A and in 7 patients (6.7%) in group B (P=.52). In all patients with AT, a NFINS was used for mapping. Table 2 shows the approach to each of the substrates.
Substrate ablation procedures performed with fluoroscopy, near zero fluoroscopy, or zero fluoroscopy
| Ablations with fluoroscopy (n=58) | Ablations with near zero fluoroscopy (n=35) | Ablations with zero fluoroscopy (n=27) | P | |
|---|---|---|---|---|
| INT | 16 (27.5) | 1 (2.8) | 8 (29.6) | .003 |
| AT (FAT or MRAT) | 0 (0) | 4 (11.4) | 2 (7.4) | .013 |
| Right AP | 12 (20.6) | 3 (8.5) | 13 (48.1) | .002 |
| Right hidden AP | 4 (6.9) | 3 (8.5) | 4 (14.8) | .506 |
| Left AP | 12 (20.6) | 13 (37.1) | 0 | <.001 |
| Left hidden AP | 14 (24.1) | 11 (31.4) | 0 | .002 |
AP, accessory pathway; AT, atrial tachycardia; FAT, focal atrial tachycardia; INT, intranodal tachycardia; MRAT, macroreentrant atrial tachycardia.
Data are expressed as No. (%).
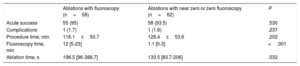
No significant differences were found in the percentage of acute success (95% in group A and 93.5% in group B; P=.53). Neither were significant differences found between groups in the percentage of complications (group A, 1.7%; group B, 1.6%). In group A there was a first-degree atrioventricular block during radiofrequency ablation of a medioseptal AP that did not require treatment, and in group B there was a patient with hypertrophic cardiomyopathy who experienced mild pericardial effusion without hemodynamic repercussions or the need for drainage during the ablation of a left posterior hidden AP (P=.23) (table 3).
Comparison of procedure, fluoroscopy, and ablation times and success, recurrence, and complication rates in both groups
| Ablations with fluoroscopy (n=58) | Ablations with near zero or zero fluoroscopy (n=62) | P | |
|---|---|---|---|
| Acute success | 55 (95) | 58 (93.5) | .530 |
| Complications | 1 (1.7) | 1 (1.6) | .237 |
| Procedure time, min | 116.1±50.7 | 128.4±53.6 | .202 |
| Fluoroscopy time, min | 12 [5-23] | 1.1 [0-3] | <.001 |
| Ablation time, s | 196.5 [96-388.7] | 133.5 [83.7-206] | .032 |
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Use of the NFINS reduced fluoroscopy time (median 1.1 [0-3] vs 12 [5-23] minutes) (P <.0005) and ablation time (median 96.5 [96-388.7] vs 133.5 [83.7-206] seconds) (P=.03) (figure 1 and table 3) without significant differences in procedure time (116.1±50.7minutes in group A vs 128.4±53.6minutes in group B; P=.2). Figure 2 shows fluoroscopy time for each substrate in both groups. When cryoablation was used, median fluoroscopy time was 6.25minutes [3.6-12.7] in group A vs 0 [0-2.2] minutes in group B.
Recurrences were analyzed by excluding unsuccessful procedures (7 cases in 4 patients), thus reducing the final analysis to 113 procedures.
Mean follow-up was longer in group A (40.9±21.6 months) than in group B (56.5±13.8 vs 26.3±16.8 months; P <.0005).
All recurrences after successful ablation occurred in the first 6 months and recurrence rates were similar in both groups: 4 out of 55 (7.3%) in group A and 4 out of 59 (6.9%) in group B (P=.61).
Recurrence rates after cryoablation were similar to those after radiofrequency ablation: there was only 1 (10%) in the cryoablation group and 7 out of 103 (6.79%) in the radiofrequency group (P=.535).
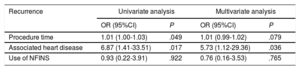
Predictors of recurrenceUnivariate analysis showed a higher rate of recurrence in patients with heart disease (odds ratio [OR], 6.87; 95% confidence interval [95%CI], 1.41-33.51; P=.017) and after ablations with longer procedure times (OR, 1.01; 95%CI, 1.00-1.03; P=.049).
Multivariate analysis (table 4) was performed to establish predictors of recurrence and the presence of structural heart disease was the only variable independently associated with recurrence after ablation (OR, 5.73; 95%CI, 1.12-29.36; P=.036).
Univariate and multivariate analysis. Factors associated with recurrence in the first 6 months after ablation
| Recurrence | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Procedure time | 1.01 (1.00-1.03) | .049 | 1.01 (0.99-1.02) | .079 |
| Associated heart disease | 6.87 (1.41-33.51) | .017 | 5.73 (1.12-29.36) | .036 |
| Use of NFINS | 0.93 (0.22-3.91) | .922 | 0.76 (0.16-3.53) | .765 |
95%CI, 95% confidence interval; OR, odds ratio; NFINS, nonfluoroscopic intracardiac navigation system.
This study presents the results of ablation in a consecutive series of pediatric patients in a regional tertiary referral center over a 6-year period. There were very high success rates and low complication rates. The results confirm that the use of NFINSs in the pediatric population for ablation with near zero or zero fluoroscopy is safe and effective, with success and complication rates similar to those of fluoroscopy-guided ablation. This technique also offers several advantages over the conventional technique in that it was associated with a significant reduction in ablation and ionizing radiation exposure times.
Ablation in pediatric patientsThese results are similar to those published in another series in the pediatric population.22–25 Although catheter ablation in pediatric patients is currently the treatment of choice,2,26 the number of ablation procedures in Spain remains low. In 2018, a total of 353 pediatric ablations were performed in Spain, (2.1% of all ablations). Furthermore, these ablations were performed in a total of 46 centers, despite the current recommendation to group them in referral centers that conduct many interventions per year, have experience in pediatric anesthesia and cardiovascular surgery, and are equipped with NFINSs.4 In our hospital, we perform more than 20 pediatric ablations per year out of a total of around 400 ablations, which are similar to the figures reported in most recent European series,18,27 and we have implemented the systematic use of NFINS and procedures without fluoroscopy in adults and children.
Ablation in children with near zero or zero fluoroscopyThis study demonstrates the safety, efficacy and feasibility of performing ablations with near zero or zero fluoroscopy in the pediatric population. The use of this technique is more widespread in adult ablations. In pediatric ablation procedures, NFINSs are used in only 20% of patients17,18 even though the risk associated with radiation exposure is greater in children than in adults.9 This fact does not have an easy explanation. It may simply be that there is little awareness of this issue and that long fluoroscopy times are assumed in children: thus, a mean radiation time <30minutes is one of the quality criteria for centers to be recognized as Centers, Services, and Referral Units (CSURs) by the Spanish National Health System.28
From 2015 onward, we decided to systematically choose a near zero or zero fluoroscopy strategy using NFINSs. This decision was based on the ALARA principle and taken after the learning curve was complete. The feasibility and efficacy of this approach was demonstrated, with results comparable to those of the conventional technique in the ablation of INT and common atrial flutter.13,29,30
This study extends this experience to ablation in the pediatric population, which is particularly vulnerable to the stochastic effects of ionizing radiation. When compared with the conventional treatment group, we found that near zero or zero use fluoroscopy significantly reduced exposure times to ionizing radiation during the ablation procedure, without prolonging procedure times. Thus, the severe consequences associated with radiation were minimized, including the life-time risk of cancer31, a period that is logically longer for children. The systematic use of NFINSs also benefits health care staff by reducing the adverse impact of the accumulated radiation dose over their professional career and by preventing the risk of severe spinal injuries associated with wearing lead aprons during these procedures.32
The results at 6-months of follow-up showed that the success, complications, and recurrence rates in both groups were similar in both groups.
Ablation time was lower in the group undergoing near zero or zero fluoroscopy. This result is in line with those of previous studies in the adult and pediatric population.33
As far as we know, this is the first study to report reductions in ablation time using NFINS-guided ablation in the pediatric population. We believe that this finding is relevant, because shorter ablation times would lead to smaller lesions in the endocardium: it has been found that myocardial tissue is still developing in children and that lesions undergo enlargement as they grow,34 with potential adverse effects. This finding is another reason in favor of the greater safety of near zero or zero fluoroscopy and should encourage its incorporation as a routine technique in all hospitals that perform ablations in the pediatric population.35
The safety and efficacy results are consistent with those of previous studies, whether the ablation procedures are guided by fluoroscopy or not.7,18,36 Our series had a high percentage of acute success and a low recurrence rate. The presence of structural heart disease was independently associated with recurrence, a finding that has already been reported in the literature.18
LimitationsThis study has a retrospective design with the limitations inherent to this approach: nevertheless, all consecutive cases were included. During the study period, no ventricular arrhythmia ablation procedures were performed in pediatric patients in our hospital. Follow-up in the near zero or zero fluoroscopy group was shorter than in the conventional treatment group. This result prevents us from being certain about long-term recurrence: however, recurrence was early in the fluoroscopy group (before 6 months of follow-up), and so it is unlikely that there would be late recurrence in the near zero or zero fluoroscopy group. Few procedures were performed with cryoablation, which could explain why the recurrence rate was lower in this group than in the group undergoing radiofrequency ablation. In this series of consecutive cases, the transseptal approach was never used for left substrate ablation, and so the results cannot be extrapolated to ablation procedures that use this route.
CONCLUSIONSThe use of NFINS-guided ablation in pediatric patients with high-risk APs or SVT is feasible and significantly reduces fluoroscopy and ablation times without increasing procedure time. Procedural success and complication rates were similar to those of fluoroscopy-guided ablation.
The use of this technique in pediatric patients also reduces ionizing radiation exposure time. Its widespread use in pediatric ablations could reduce the risk of ionizing radiation.
CONFLICTS OF INTERESTNone declared.
- –
Catheter ablation with near zero or zero fluoroscopy has been shown to decrease fluoroscopy and ablation times. Its efficacy and safety profiles are similar to those of fluoroscopy-guided ablation in the adult population.
- –
However, there are few studies on the use of this technique in the pediatric population.
- –
This study demonstrates that ablation with near zero or zero fluoroscopy are feasible in pediatric patients.
- –
Ablation with near zero or zero fluoroscopy decreases fluoroscopy and ablation times with success and complication rates comparable to those of fluoroscopy-guided ablation.
- –
The results are comparable to those of the adult population.