Renal denervation is a percutaneous intervention for the treatment of resistant hypertension. Randomized studies have shown contradictory results on its efficacy. We present the results of a renal denervation registry for the treatment of resistant hypertension in real-life patients in Spain.
MethodsMulticenter registry of consecutive patients with resistant hypertension treated with renal denervation in Spain between 2009 and 2018.
ResultsWe included 125 patients (mean age, 56 years; 41% female; mean onset of hypertension 14±9 years previously). Office systolic and diastolic blood pressure and ambulatory blood pressure monitoring decreased 6 months after the intervention (166±20/95±16 to 149±22/87±16 mmHg and 151±14/89±12 to 143±15/84±11, both P <.0001). At 12 months, the blood pressure reduction was maintained and the number of antihypertensive drugs decreased from 4.9±1.2 to 4.4±1.5 (P=.0001). There were no significant procedure-related complications. The response rate to denervation at 1 year was 80%, but there were wide differences between centers.
ConclusionsIn patients with resistant hypertension, treatment with renal denervation was related to a decrease in office blood pressure and, more importantly, in ambulatory blood pressure monitoring, with a significant reduction in pharmacological treatment.
Keywords
Hypertension is the most prevalent modifiable cardiovascular risk factor, affecting more than 1 billion people worldwide and causing the death of more than 9 million people each year.1 Observational studies have revealed a progressive association between elevated blood pressure (BP) and the development of cardiovascular disease,2 with an increase of 20mmHg in systolic BP and of 10mmHg in diastolic BP, doubling the risk of cardiovascular death.3
Despite efforts to control the condition, data from 2010 show that one-third of individuals with hypertension in developed countries were unaware of having the disease, slightly more than half received drug therapy, and less than a third had adequate pressure control.4 Due to the limited ability of drug therapy to normalize BP in a significant number of patients, various nonpharmacological options have emerged in recent years for the management of hypertension. One such approach is percutaneous renal denervation (pRDN), a treatment based on modulation of the sympathetic nervous system. The renal sympathetic nervous system is involved in the development and progression of hypertension and in the organ damage caused by the condition. Afferent sympathetic nerve fibers projecting to the central nervous system are activated in the kidney and in response to different local stimuli; this activation increases the sympathetic tone and thereby elevates BP by increasing cardiac contractility, promoting vasoconstriction, and stimulating the renin-angiotensin-aldosterone axis and renal sodium retention, all mediated by efferent sympathetic pathways.5
pRDN is a minimally invasive procedure involving the introduction of a catheter into the renal artery that, by releasing energy (typically, radiofrequency energy), produces a lesion in the afferent and efferent nerves of the sympathetic nervous system surrounding the renal arteries.6 Interruption of afferent renal sympathetic pathways decreases BP by reducing vascular tone and cardiac contractility while increasing natriuresis, whereas interruption of efferent renal sympathetic pathways decreases the activation of the renin-angiotensin-aldosterone system and sodium retention.5
Despite the initial expectations generated by the first published studies, which showed improved BP values after radiofrequency-based pRDN in patients with resistant hypertension,7,8 pRDN failed to improve BP vs a sham procedure in the SYMPLICITY HTN-3 study.9 This unexpected result did not correspond to those of previous studies7,8 or the data derived from real-world registries,10 but still led to an almost complete abandonment of the technique. However, several recent studies conducted in a small number of patients with moderate hypertension have shown that pRDN effectively improves both office BP and, more importantly, 24-hour ambulatory BP monitoring (ABPM) levels in both patients without drug therapy and patients with incomplete treatment.11–13
In Spain, pRDN was introduced at the same time as in other European countries and Australia for the treatment of resistant hypertension, although its penetration was significantly lower. In 2014, a working group on renal denervation was established with the aim of sharing experiences with this new technique for the treatment of hypertension. We present data from a retrospective multicenter registry of patients with resistant hypertension who underwent pRDN with a unipolar (SYMPLICITY FLEX, Medtronic Inc) or tetrapolar (SYMPLICITY SPYRAL, Medtronic Inc) radiofrequency catheter between 2009 and 2018.
METHODSThis multicenter registry with retrospective data collection included consecutive patients with resistant hypertension who underwent pRDN with SYMPLICITY FLEX (2009-2015) or SPYRAL (2015-2018) catheter in 7 Spanish centers.
Data collectionThe data included in the registry were retrospectively obtained from analysis of the local databases in each center or from patients’ clinical records and include baseline clinical data and office BP and ABPM values before the procedure and during a 12-month follow-up after the pRDN.
PatientsWe included consecutive patients with resistant hypertension treated with pRDN. Resistant hypertension was defined as office BP higher than 140/90mmHg despite stable drug therapy for a minimum of 4 weeks with 3 or more antihypertensive drugs, one of which had to be a diuretic.13
All centers participating in the registry had units specialized in the management of hypertension. By protocol, secondary causes of resistant hypertension were ruled out before the indication for pRDN using clinical records, physical examination, renal imaging, endocrinological screening, and polysomnography. Patients with a known secondary cause of resistant hypertension were not considered candidates for pRDN. ABPM was not deemed necessary for the pRDN indication, but its results were recorded when available. No information is available on patients assessed for pRDN who were ruled out because they were not taxpayers.
Blood pressure measurementsOffice BP was determined according to the recommendations of the clinical practice guidelines of the European Society of Cardiology and the European Society of Hypertension.14 ABPM was performed in accordance with current recommendations, and the mean systolic BP and diastolic BP values were evaluated over 24hours.15
Renal denervation procedureThe pRDN procedure was performed in accordance with the recommendations of the device manufacturer6 by interventional cardiologists, vascular surgeons, or electrophysiologists, as standard in each center. Between 2009 and 2015, the procedure was performed with the SYMPLICITY FLEX monopolar device (Medtronic Inc); from 2015, it was performed with the SYMPLICITY SPYRAL tetrapolar device (Medtronic Inc). Patients were considered to be responders when the 12-month systolic BP decreased by a minimum of 10mmHg or when the 24-hour systolic BP decreased by a minimum of 5mmHg.
Statistical analysisBP values during follow-up were compared using a Student t test for paired data. Mean BP values are expressed as the 95% confidence interval (95%CI). P <.05 was considered statistically significant. Statistical analyses were performed with Stata 15IC (Stata Corp, College Station, Texas, United States).
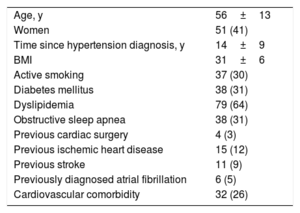
RESULTSStudy populationDuring the study period, pRDN was performed in 125 patients. The baseline characteristics of the patients included in the registry are summarized in table 1.
Baseline clinical characteristics of the 125 patients with resistant hypertension treated with pRDN
| Age, y | 56±13 |
| Women | 51 (41) |
| Time since hypertension diagnosis, y | 14±9 |
| BMI | 31±6 |
| Active smoking | 37 (30) |
| Diabetes mellitus | 38 (31) |
| Dyslipidemia | 79 (64) |
| Obstructive sleep apnea | 38 (31) |
| Previous cardiac surgery | 4 (3) |
| Previous ischemic heart disease | 15 (12) |
| Previous stroke | 11 (9) |
| Previously diagnosed atrial fibrillation | 6 (5) |
| Cardiovascular comorbidity | 32 (26) |
BMI, body mass index.
Values represent No. (%) or mean±standard deviation.
Cardiovascular comorbidity was considered present when the patient had at least 1 of the following: heart failure, ischemic heart disease, stroke, or atrial fibrillation.
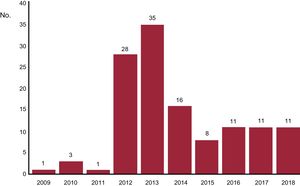
The pRDN procedure was successfully completed in the 125 patients. No data were collected on the anatomy of the renal arteries, presence of polar arteries, or number or location of radiofrequency ablations. In all patients, the procedure was performed via femoral access. There were no immediate renal complications related to the intervention and only 2 patients had a puncture site-related complication (2 incidences of femoral pseudoaneurysm). There was no worsening of renal function related to the procedure. The distribution of patients throughout the years of the registry is shown in figure 1. It should be noted that the initial patients treated between 2009 and 2011 were included in the SYMPLICITY HTN-1 and SYMPLICITY HTN-2 studies. The procedure was performed with the SYMPLICITY FLEX monopolar catheter in 85 patients (68%) and with the SYMPLICITY SPYRAL catheter in the remaining 40.
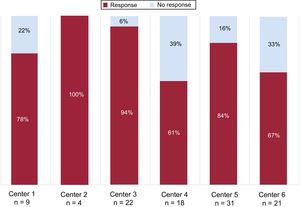
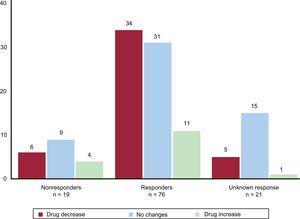
The overall response rate to the pRDN was 80.6%; the response rate was 79.6% in patients treated with the SYMPLICITY FLEX catheter and 80% in those treated with the SYMPLICITY SPYRAL catheter. There was considerable variability in the response rate among centers (figure 2).
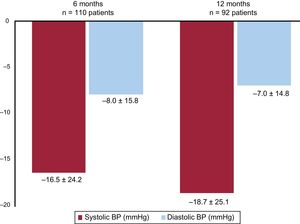
Office blood pressure valuesOffice BP measurements during the study period are presented in table 2; the changes in office BP during follow-up are shown in figure 3. Systolic BP and diastolic BP were decreased at 6 months after the pRDN (from 166±20 to 149±22mmHg and from 95±16 to 87±16mmHg, respectively; both P <.0001). The distributions of systolic office BP before the pRDN and at 6 and 12 months of follow-up are shown in figure 4.
Changes over time in office BP
| Baselinen=110 | 6 mon=110 | P | Baselinen=92 | 12 mon=92 | P | |
|---|---|---|---|---|---|---|
| Systolic office BP, mmHg | 166±20(95%CI, 162-170) | 149±22(95%CI, 145-154) | <.0001 | 165±20(95%CI, 160-169) | 146±22(95%CI, 141-151) | <.0001 |
| Diastolic office BP, mmHg | 95±16(95%CI, 92-98) | 87±16(95%CI, 84-90) | <.001 | 94±16(95%CI, 91-97) | 87±16(95%CI, 84-90) | <.0001 |
95%CI, 95% confidence interval; BP, blood pressure.
Analysis of the changes over time in office BP values in 110 patients with follow-up at 6 months and in 92 patients with follow-up at 12 months. BP is expressed as mean±standard deviation with the 95% confidence interval. P value vs baseline values.
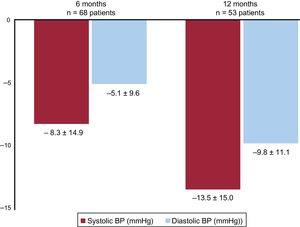
ABPM values during the study period are presented in table 3; the changes in ABPM levels during follow-up are shown in figure 5. The systolic BP and diastolic BP on ABPM were decreased at 6 months after the pRDN (from 151±14 to 143±15mmHg and from 89±12 to 84±11mmHg, respectively; both P <.0001).
Changes over time in 24-hour ABPM values
| Baselinen=68 | 6 mon=68 | P | Baselinen=53 | 12 mon=53 | P | |
|---|---|---|---|---|---|---|
| Systolic 24-h BP, mmHg | 151±14(95%CI, 148-154) | 143±15(95%CI, 148-154) | <.0001 | 150±14(95%CI, 146-154) | 136±16(95%CI, 132-141) | <.0001 |
| Diastolic 24-h BP, mmHg | 89±12(95%CI, 86-92) | 84±11(95%CI, 82-87) | <.0001 | 92±12(95%CI, 89-95) | 82±14(95%CI, 78-86) | <.0001 |
95%CI, 95% confidence interval; ABPM, 24-hour ambulatory blood pressure monitoring; BP, blood pressure.
Analysis of the changes over time in systolic and diastolic BP values on 24-hour ABPM in 68 patients with follow-up at 6 months and in 53 patients with follow-up at 12 months. BP is expressed as mean±standard deviation (95%CI). P value vs baseline values.
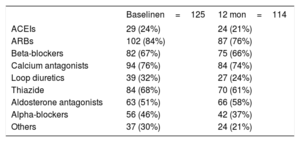
The drug therapy at the time of denervation and at 12 months of follow-up is shown in table 4. The number of antihypertensive drugs decreased from 4.9±1.2 before the pRDN to 4.4±1.5 at 12 months of follow-up (P = .0001). Figure 6 shows the relationship between the treatment changes during follow-up (increase, decrease, or no change) and the response to pRDN (response, no response, or impossible to measure because there were no BP or ABPM data). The only drugs whose prescription increased at follow-up were aldosterone antagonists. In total, 21% of the 19 nonresponders were on aldosterone antagonist therapy before denervation vs 55% of the 76 responders. During follow-up, this therapy increased to 37% in nonresponders (discontinuation in 1 patient and initiation in 4) and to 57% in responders (discontinuation in 6 patients and initiation in 7).
Changes over time in drug therapy during the study
| Baselinen=125 | 12 mon=114 | |
|---|---|---|
| ACEIs | 29 (24%) | 24 (21%) |
| ARBs | 102 (84%) | 87 (76%) |
| Beta-blockers | 82 (67%) | 75 (66%) |
| Calcium antagonists | 94 (76%) | 84 (74%) |
| Loop diuretics | 39 (32%) | 27 (24%) |
| Thiazide | 84 (68%) | 70 (61%) |
| Aldosterone antagonists | 63 (51%) | 66 (58%) |
| Alpha-blockers | 56 (46%) | 42 (37%) |
| Others | 37 (30%) | 24 (21%) |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers.
Relationship between the changes in treatment during follow-up (increase, decrease, or no change) and the response to pRDN (response, without response, or impossible to measure due to a lack of BP or ABPM data). ABPM, 24-hour ambulatory blood pressure monitoring; BP, blood pressure; pRDN, percutaneous renal denervation.
Analysis of the data from this multicenter registry of patients with resistant hypertension treated with pRDN in a real-life setting has shown that the treatment effectively decreases office BP and, more importantly, ABPM values, with a reduction in the number of antihypertensive drugs taken by the patients. The procedure was safe, with a very low rate of intervention-related complications. The success of the intervention was independent of the type of radiofrequency catheter used, although variability in the response rate per center was observed.
The fall in BP values shown in our registry is in agreement with that obtained in previous studies with real-world data.10,16–19 In addition, the BP improvement was also seen in the 24-hour ABPM. This result is particularly important because a recent study showed that it is a better predictor of cardiovascular mortality than office BP.20 In addition, this improvement correlated with a decrease in the number of drugs taken, in contrast to previous registries that showed no significant decrease in drug therapy.10,16–19 The only drugs whose prescription increased were aldosterone antagonists. These drugs were established with a I B level of recommendation as a fourth-line treatment for patients with resistant hypertension in the latest 2018 European guidelines for the treatment of hypertension.14 Thus, it is notable that only 51% of the denervation candidates were being treated with these drugs. However, this recommendation was more controversial in the previous guidelines from 2013, which awarded it a IIa B level recommendation,21 and our series includes patients from 2009. In addition, the pRDN response was significantly worse in patients not taking aldosterone antagonists. Nowadays, candidates for pRDN should be under treatment with aldosterone antagonists.
Despite the promising results of the initial studies of pRDN, the first study to compare pRDN with a sham pRDN procedure in patients with refractory hypertension, the SYMPLICITY HTN-3 trial, showed no benefit of treatment on office or ambulatory BP.9 The results of that study have been questioned since its publication22 and, in a post hoc analysis, Kandzari et al.23 determined a lower than expected response in the group treated with pRDN and a much higher than expected response in the control group vs previous experiences. This analysis revealed potential confounding factors that could explain, at least partly, the unexpected results, such as operator inexperience, the inclusion of patients with isolated systolic hypertension, poor adherence to drug therapy before selection and during the study, inclusion of a high percentage of African-American patients, and procedural deficiencies in terms of ablation number and location.23 Regarding the procedure, reasonable doubts arose about whether the pRDN was performed in the same way as in the first studies, given that the GLOBAL SYMPLICITY Registry, with experienced operators and in a similar population, calculated a mean systolic BP fall of 19.3±22.4mmHg at 6 months with a greater number of ablations per patient (13.5±4.1).10 A lack of ablations in the 4 quadrants and the number of radiofrequency ablations were correlated with the reduction in BP.23 Our registry has no procedural information but does highlight the heterogeneity in the pRDN response rate among centers. This finding, consistent with that observed in the SYMPLICITY HTN-3 study, most likely indicates the importance of good patient selection and of performing as meticulous a procedure as possible. In this regard, the indication for pRDN should always be based on the ABPM results to exclude pseudoresistance or white-coat hypertension, after all secondary causes of resistant hypertension have been ruled out, with a medication adjustment in line with the guidelines and stable medication for at least 4 weeks before the denervation. Regarding the intervention, the SYMPLICITY HTN-3 trial was criticized because more than 50% of the patients included were treated by operators who were performing their first or second procedure, suggesting that the learning curve might have influenced the intervention outcomes.23 In our registry, the procedures were performed by the same operators (1 or 2 per center), with a volume equal to or higher than that in the SYMPLICITY HTN-1 or SYMPLICITY HTN-2 studies and much higher than in the SYMPLICITY HTN-3 trial. Currently, with what has been learned after the analysis of previous studies and taking into account the anatomy of the sympathetic plexus at the kidney level,24 the intervention objectives should be to treat the 4 quadrants of the renal artery, with the maximum number of radiofrequency ablations possible (leaving a safe 5-mm distance between applications), as well as the main renal artery and its branches (with a diameter> 3mm).6 Unexpectedly, there was no difference in the efficacy of the intervention between the SYMPLICITY FLEX and SYMPLICITY SPYRAL catheters, even though the latter was designed to improve the efficacy of the procedure.6
The results of this registry reinforce the role of pRDN in the management of hypertension and, even though the indication for pRDN was drastically reduced after the publication of the SYMPLICITY HTN-3 trials, the recently published SPYRAL HTN ON-MED, SPYRAL HTN OFF-MED, and RADIANCE SOLO studies have provided the first consistent evidence on the potential clinical usefulness of pRDN in the treatment of patients who cannot or do not want to take antihypertensive drugs. These studies included patients with moderate hypertension, with nonoptimized drug therapy or without treatment, and a control group with pRDN sham and identified significant decreases in office BP and ABPM values in the treated patients, although the number of patients included was small and the follow-up short.11–13 In addition, a recent meta-analysis of pRDN included 977 patients from 6 clinical trials, including the SYMPLICITY HTN-3 study: compared with a sham control group, pRDN improved BP control in treated patients.25 These results should be confirmed in studies that are already underway and are likely to change the approach to the treatment of hypertension in the coming years.26 In this regard, one of the most novel findings of our registry vs previous ones is that pRDN can not only improve BP control, but also simultaneously reduce pharmacological treatment.
LimitationsDue to the observational and retrospective nature of this study, the results presented are subject to limitations. The intervention indication criteria were not standardized and could have varied among the different centers. In some patients, BP or ABPM records were not available during follow-up and some of the included patients have not yet completed 12 months of follow-up. The lack of procedural data, such as the anatomy of the renal arteries, presence of untreated polar arteries, number of radiofrequency ablations, treatment of the main branches alone or also of the renal artery branches, limit the analysis of the possible causes of the lack of response to pRDN in 20% of the included patients, as well as the analysis of the response variability among the different centers. The lack of a control group does not allow a clear and direct relationship between pRDN and improved BP figures to be established, which could be due to a placebo effect, a Hawthorne effect, or a regression to the mean and could lead to overestimation of the effect of the pRDN,27 although the results are in line with those of other studies performed after the SYMPLICITY HTN-3 trial.
CONCLUSIONSIn real-world patients with resistant hypertension treated with radiofrequency-based pRDN, there is a fall in office BP and, more importantly, 24-hour ABPM values in up to 80% of patients, with a significant decrease in drug therapy. The procedure is safe, with a very low rate of intervention-related complications. The pRDN response is independent of the type of radiofrequency catheter used, although there is significant variability in the response rate per center.
CONFLICTS OF INTERESTNone.
- –
Renal denervation is an intervention for the treatment of resistant hypertension that has shown contradictory efficacy results.
- –
Three recent randomized studies with a small number of patients and short follow-up showed that denervation was effective in the control of moderate hypertension in patients without pharmacological treatment or with incomplete treatment.
- –
This work shows the denervation results of real-world patients with resistant hypertension in Spain, with a significant improvement in both office and ambulatory BP.
- –
The improvement in BP values is additionally accompanied by a decrease in the number of antihypertensive drugs being taken.
- –
There is wide variability among centers in the response to renal denervation, which highlights the importance of adequate patient selection and of as meticulous an intervention as possible.
.