Study of inherited heart diseases (IHD) involves performing diagnostic tests, which are sometimes inconvenient or stressful, in asymptomatic relatives. The aim of this study was to analyze refusal to undergo various diagnostic tests and follow therapeutic recommendations.
MethodsWe assessed 1992 consecutive families with IHD to analyze refusal to undergo family screening. The study included 1539 individuals who were recommended to undergo cardiac magnetic resonance, and 837 who were recommended a drug challenge test. To study treatment refusal, we assessed 395 patients with an indication for an implantable cardioverter-defibrillator (ICD) and 402 patients with an indication for anticoagulation.
ResultsA total of 28% of families who were recommended to undergo screening for suspected IHD did not attend, but refusal was lower if there was a family history of sudden cardiac death. In all, 23% did not undergo magnetic resonance, and the 2 main reasons were administrative problems (53%) and claustrophobia (18%). Refusal was more common in older people, women, symptomatic persons, individuals with arrhythmias, and relatives. Nearly one fifth (19%) did not take the drug challenge test, due to fear (46%) or administrative issues (25%). Refusal was more frequent in older individuals, asymptomatic persons, those with a history of arrhythmias, relatives, and those with a positive genetic study. Only a minority of patients rejected the treatments (5.1% ICD, 2.5% anticoagulation). The percentage of sudden cardiac death in persons rejecting ICD implantation was high (4.5% per year).
ConclusionsOne fifth of people attending screening for IHD refused to undergo more sophisticated and stressful tests. This study identified several independent predictors associated with refusal. Only a minority of high-risk patients refused treatments such as ICD implantation and anticoagulation.
Keywords
Inherited heart diseases are heart conditions that affect different members of the same family. They include cardiomyopathies (CMs)—mainly hypertrophic CM (HCM), dilated CM (DCM), and arrhythmogenic CM (ACM)—and channelopathies—mainly long QT syndrome (LQTS) and Brugada syndrome. The prevalence of IHD in Europe ranges from 1 case per 500 population for HCM to 1 case per 2000 population for channelopathies. An estimated 150 000 people in Spain have an IHD.1
IHDs have a number of common characteristics. First, they have a genetic basis, affect different members of the same family,2 and are typically inherited as an autosomal dominant trait. Second, they can cause sudden cardiac death (SCD) without prior symptoms. Third, they are progressive conditions with a heterogeneous clinical presentation, and in many cases incomplete penetrance,1–3 that cause significant arrhythmic (atrial and ventricular) morbidity and mortality.4 Atrial fibrillation, for example, affects up to 30% of patients with HCM and DCM.5 Patients with CMs can also develop heart failure requiring transplantation.4
A complete cardiac evaluation can identify at-risk individuals, who can be effectively protected against future complications with medications, interventions, and cardiac devices.
The main aim of this study was to calculate the proportion of families and patients who refuse recommended diagnostic tests and treatments for IHD and to identify associated characteristics and reasons for refusal. We defined 3 specific objectives:
Objective 1: To calculate the proportion of families who refuse cardiac screening, determine their reasons for refusal, and assess the diagnostic yield of screening for suspected IHD in families.
Objective 2: To calculate the proportion of relatives who refuse cardiac magnetic resonance imaging (MRI) or a pharmacological challenge and determine their reasons for refusal.
Objective 3: To calculate the proportion of patients with CMs or channelopathies who refuse to undergo implantable cardioverter-defibrillator (ICD) implantation or receive anticoagulation therapy and determine the medium-term consequences of this decision.
MethodsWe conducted a retrospective cross-sectional study of prospectively recorded cases in the database of a dedicated IHD unit between 2003 and 2017 (figure 1 of the supplementary data). To determine reasons for not attending cardiac MRI or pharmacological challenge appointments, we conducted a telephone survey of randomly selected individuals.
PopulationObjective 1: To calculate the proportion of families who did not show up for the first stage of cardiac screening (basic evaluation), we analyzed 1992 families: 1662 with CMs and 330 with channelopathies. In the first group, there were 1087 families (65.4%) with HCM, 434 (26.1%) with DCM, and 141 (8.5%) with ACM. In the second group, there were 238 families (72.1%) with Brugada syndrome and 92 (27.9%) with LQTS.
Objective 2: To calculate the proportion of patients who did not show up for cardiac MRI, we studied 1539 patients (mean±standard deviation [SD] age, 47±7 years; 48% women) in whom this test was considered advisable after an electrocardiogram and an echocardiogram. The family condition was HCM in 889 cases (57.8%), DCM in 406 (26.4%), and ACM in 244 (15.8%). We then interviewed 102 patients (52 women and 50 men) who did attend an appointment to determine their reasons. To calculate the proportion of patients who did not undergo the pharmacological challenge, we studied 837 patients (mean±SD age, 38.7±16 years, 54% men) for whom provocative testing with a sodium channel blocker or adrenaline had been requested. In this case, the family condition was Brugada syndrome in 741 cases (88.5%) and LQTS in 96 (11.5%). To investigate reasons for refusal, we interviewed 92 patients (49 women and 43 men).
Objective 3: For objective 3, we selected high-risk patients with an indication for ICD implantation (n=395) or long-term anticoagulation (n=402) from a group of 2372 patients with a definitive diagnosis of a CM or a channelopathy (43% HCM, 18% DCM, 7% ACM, 10% Brugada syndrome, 4% LQTS, 18% other). We then calculated the proportion of patients who refused these treatments and investigated complications associated with this decision.
Statistical analysisThe statistical analysis was performed in SPSS (version 20.0). The data were analyzed using basic descriptive statistics, with qualitative variables expressed as percentages and quantitative variables as mean±SD.
To analyze demographic and clinical predictors of refusal to follow medical recommendations, we studied the following variables: type of IHD (CM, channelopathy, and subtypes), age, sex, family history of SCD, presence of symptoms or arrhythmias, proband status, genetic study (yes/no), a positive genetic test result, and confirmation of IHD in the family.
Dichotomous qualitative variables were compared using Pearson's χ2 test and quantitative variables using the t test for equality of variances. Logistic regression analysis with backward elimination was used to evaluate associations between individual variables and refusal of diagnostic tests, without adjustment for potential confounders. In all cases, differences were considered significant for a P value of less than .05.
ResultsProportion of families who did not undergo cardiac screening and reasons for refusalA total of 472 families with a CM (28.4%) did not undergo cardiac screening after the initial evaluation of the proband and family tree. There were 287 families (26.4%) with HCM, 151 (34.8%) with DCM, and 34 (24.1%) with ACM. There were significant differences in refusal rates between DCM and HCM (P=.001) and between DCM and ACM (P=.018). Refusal was highest in families with DCM.
Seventy-eight families with a channelopathy (23.6%) did not participate in the cardiac screening program: 51 (21.4%) with Brugada syndrome and 27 (29.3%) with LQTS. No significant differences were found between families with Brugada syndrome and those with LQTS (P=.129).
We did, however, observe significant differences between families with and without a history of SCD (21.0% vs 29.7%, P<.005). Refusal rates were lower in families with multiple cases of SCD (10.3% vs 29.7%, P<.0001).
The main reason listed for failure to attend the basic screening evaluation was “unknown/did not show up” (324 families [58.9%]). Other reasons were “administrative problems” (149 families [27.1%]), “residence in another region or country” (45 families [8.2%]), “direct refusal with no explanation” (14 families [2.5%]), and”wants to postpone appointment” (11 families [2.0%]).
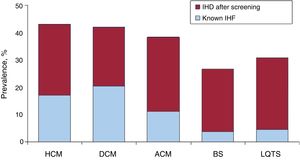
Diagnostic yield of family screeningJust 215 (14.9%) of the 1442 families who participated in the screening program had a confirmed diagnosis of IHD before screening. This number increased to 574 (39.8%) after screening (figure 1).
Prevalence of different forms of IHD before and after family screening at an inherited heart disease unit. ACM, arrhythmogenic cardiomyopathy; BS, Brugada syndrome; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; IHD, inherited heart disease; LQTS, long QT syndrome.
Significant differences for a confirmed diagnosis were observed between CMs and channelopathies (42.4% vs 27.8%, P<.0005). There were no differences between the different subtypes of CMs or channelopathies.
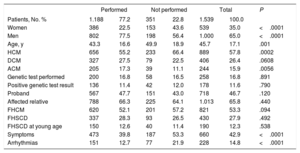
Refusal of second-line testingReasons for refusing cardiac MRIIn total, 351 patients (22.8%) who were advised to undergo cardiac MRI chose not to. Compared with patients who agreed to the procedure, these individuals were significantly older (49.9±18.9 vs 43.3±16.6 years, P=.001) and more likely to be women (28.4% vs 19.8%, P<.0005). Significant differences were found between HCM (26.2%) and the other forms of CM (19.5% and 16.0%, P=.0001). Refusal was highest in the HCM group. The main characteristics of the patients who chose not to undergo cardiac MRI are summarized in table 1.
Characteristics of patients who refused to undergo cardiac magnetic resonance imaging
| Performed | Not performed | Total | P | ||||
|---|---|---|---|---|---|---|---|
| Patients, No. % | 1.188 | 77.2 | 351 | 22.8 | 1.539 | 100.0 | |
| Women | 386 | 22.5 | 153 | 43.6 | 539 | 35.0 | <.0001 |
| Men | 802 | 77.5 | 198 | 56.4 | 1.000 | 65.0 | <.0001 |
| Age, y | 43.3 | 16.6 | 49.9 | 18.9 | 45.7 | 17.1 | .001 |
| HCM | 656 | 55.2 | 233 | 66.4 | 889 | 57.8 | .0002 |
| DCM | 327 | 27.5 | 79 | 22.5 | 406 | 26.4 | .0608 |
| ACM | 205 | 17.3 | 39 | 11.1 | 244 | 15.9 | .0056 |
| Genetic test performed | 200 | 16.8 | 58 | 16.5 | 258 | 16.8 | .891 |
| Positive genetic test result | 136 | 11.4 | 42 | 12.0 | 178 | 11.6 | .790 |
| Proband | 567 | 47.7 | 151 | 43.0 | 718 | 46.7 | .120 |
| Affected relative | 788 | 66.3 | 225 | 64.1 | 1.013 | 65.8 | .440 |
| FHCM | 620 | 52.1 | 201 | 57.2 | 821 | 53.3 | .094 |
| FHSCD | 337 | 28.3 | 93 | 26.5 | 430 | 27.9 | .492 |
| FHSCD at young age | 150 | 12.6 | 40 | 11.4 | 190 | 12.3 | .538 |
| Symptoms | 473 | 39.8 | 187 | 53.3 | 660 | 42.9 | <.0001 |
| Arrhythmias | 151 | 12.7 | 77 | 21.9 | 228 | 14.8 | <.0001 |
ACM, arrhythmogenic cardiomyopathy; DCM, dilated cardiomyopathy; FHSCD, family history of sudden cardiac death; FHCM, family history of cardiomyopathy; HCM: hypertrophic cardiomyopathy.
Cardiac magnetic resonance imaging performed versus unperformed, totals and significance. Data are expressed as No. (%) or mean ± SD.
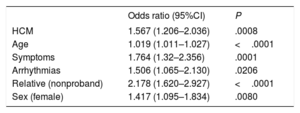
In the multivariate analysis, older people (odds ratio [OR]=1.02, 95% confidence interval [95%CI], 1.01-1.03; P<.0001), women (OR=1.42; 95%CI, 1.09-1.83; P=.008), individuals with a history of symptoms (OR=1.76, 95%CI, 1.32-2.36; P=.0001) or arrhythmias (OR=1.5, 95%CI, 1.06-2.13; P=.02), and relatives (vs probands) (OR=2.18; 95%CI, 1.62-2.93; P<.0001) were more likely to refuse cardiac MRI. Relatives of families with HCM were significantly more likely to refuse the test than those of families with DCM or ACM (OR=1.57, 95%CI, 1.21-2.04; P=.0008) (table 2).
Multivariate analysis of variables associated with refusal to undergo cardiac magnetic resonance imaging
| Odds ratio (95%CI) | P | |
|---|---|---|
| HCM | 1.567 (1.206–2.036) | .0008 |
| Age | 1.019 (1.011–1.027) | <.0001 |
| Symptoms | 1.764 (1.32–2.356) | .0001 |
| Arrhythmias | 1.506 (1.065–2.130) | .0206 |
| Relative (nonproband) | 2.178 (1.620–2.927) | <.0001 |
| Sex (female) | 1.417 (1.095–1.834) | .0080 |
95%CI, confidence interval; HCM, hypertrophic cardiomyopathy.
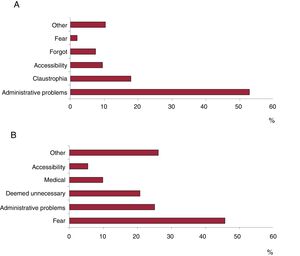
The telephone survey to investigate reasons for not undergoing cardiac MRI was conducted among 102 consecutive patients (52 women and 50 men), several of whom indicated more than 1 reason. The reasons mentioned were administrative problems (52.8%), claustrophobia (17.9%), residence in another region (8.0%), lack of interest, information, or time (8.0%), and other (10.3%). There were no significant differences in percentage distribution of reasons given by men and women (figure 2).
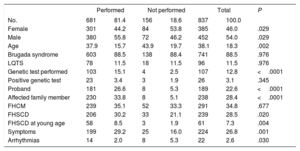
Reasons for refusing a pharmacological challengeIn total, 156 patients (18.6%) in whom a pharmacological challenge was indicated did not undergo the test. Women outnumbered men (n=84 [21.8%] vs n=72 (15.9%), P=.029) and those who refused the test were significantly older than those who agreed to it (43.9±19.7 vs 37.9±15.7 years, P=.002). The main characteristics of the patients who chose not to undergo the challenge are summarized in table 3.
Characteristics of patients who refused to undergo pharmacological challenge
| Performed | Not performed | Total | P | ||||
|---|---|---|---|---|---|---|---|
| No. | 681 | 81.4 | 156 | 18.6 | 837 | 100.0 | |
| Female | 301 | 44.2 | 84 | 53.8 | 385 | 46.0 | .029 |
| Male | 380 | 55.8 | 72 | 46.2 | 452 | 54.0 | .029 |
| Age | 37.9 | 15.7 | 43.9 | 19.7 | 38.1 | 18.3 | .002 |
| Brugada syndrome | 603 | 88.5 | 138 | 88.4 | 741 | 88.5 | .976 |
| LQTS | 78 | 11.5 | 18 | 11.5 | 96 | 11.5 | .976 |
| Genetic test performed | 103 | 15.1 | 4 | 2.5 | 107 | 12.8 | <.0001 |
| Positive genetic test | 23 | 3.4 | 3 | 1.9 | 26 | 3.1 | .345 |
| Proband | 181 | 26.6 | 8 | 5.3 | 189 | 22.6 | <.0001 |
| Affected family member | 230 | 33.8 | 8 | 5.1 | 238 | 28.4 | <.0001 |
| FHCM | 239 | 35.1 | 52 | 33.3 | 291 | 34.8 | .677 |
| FHSCD | 206 | 30.2 | 33 | 21.1 | 239 | 28.5 | .020 |
| FHSCD at young age | 58 | 8.5 | 3 | 1.9 | 61 | 7.3 | .004 |
| Symptoms | 199 | 29.2 | 25 | 16.0 | 224 | 26.8 | .001 |
| Arrhythmias | 14 | 2.0 | 8 | 5.3 | 22 | 2.6 | .030 |
FHCM, family history of cardiomyopathy; FHSCD, family history of sudden cardiac death; LQTS, long QT syndrome.
Challenge test performed versus unperformed, totals and statistical significance. Data are expressed as No. (%) or mean ± standard deviation.
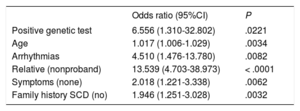
In the multivariate analysis, older individuals (OR=1.02; 95%CI, 1.01-1.03; P=.003), asymptomatic patients (OR=2.02, 95%CI, 1.22-3.34; P=.006), patients with a history of arrhythmia (atrial fibrillation or ventricular tachycardia) (OR=4.51, 95%CI, 1.48-13.78; P=.008), relatives (OR=13.54; 95%CI, 4.70-38.97; P<.0001), and patients with a positive genetic test result (OR=6.56; 95%CI, 1.31-32.80; P=.02) (table 4) were more likely to refuse a pharmacological challenge.
Analysis of variables associated with refusal of pharmacological challenge
| Odds ratio (95%CI) | P | |
|---|---|---|
| Positive genetic test | 6.556 (1.310-32.802) | .0221 |
| Age | 1.017 (1.006-1.029) | .0034 |
| Arrhythmias | 4.510 (1.476-13.780) | .0082 |
| Relative (nonproband) | 13.539 (4.703-38.973) | < .0001 |
| Symptoms (none) | 2.018 (1.221-3.338) | .0062 |
| Family history SCD (no) | 1.946 (1.251-3.028) | .0032 |
95%CI, 95% confidence interval; SCD, sudden cardiac death.
The main reason given by the 92 patients when interviewed by telephone was fear of the test and its possible complications (45.7%), followed by administrative problems (25.0%), consideration of the test as unnecessary (20.7%), having another medical condition at the time of appointment (9.8%), residence in another region (5.4%); 26.1% mentioned other reasons (figure 2). There were no significant differences in the percentage distribution of reasons given by men and women.
Refusal of recommended treatmentsRefusal to undergo ICD implantationTwenty patients (5.1%) considered to be at high risk for malignant arrhythmias refused to undergo primary ICD implantation. There was no difference in refusal rates between men and women (4.9% [n=14] vs 5.4% [n=14], P=.87). Patients who refused to undergo ICD implantation were of a similar age to those who agreed to the procedure (37.6±19.3 vs 40.2±18.8 years, P=.5).
No single condition predominated among the patients who refused to undergo ICD implantation (11 had HCM, 4 DCM, 2 ACM, and 3 Brugada syndrome). After a mean follow-up period of 3.9±2.5 years, 3 of these patients (15.0%) experienced SCD (2 had DCM and 1 had ACM).
Refusal of anticoagulation therapyTen patients considered to be at high risk for thromboembolism (2.5%) refused anticoagulant therapy: 4 (44.4%) had experienced bleeding (major in 2 cases), 8 (88.9%) were on simple antiplatelet therapy, and 1 (11.1%) was on dual antiplatelet therapy.
A similar proportion of women and men refused anticoagulation (2.8% [n=7] vs 1.9% [n=3], P=.58), and the ages of those who refused and those who agreed were similar (46.3±19.5 vs 52.0±21.3 years, P=.4). None of the 10 patients who refused anticoagulation therapy developed a thromboembolic complication over a mean follow-up period of 3.8±2.9 years.
DiscussionOur study has provided important insights into the operation of a dedicated IHD unit and is the first to determine the proportion of patients with IHD and their relatives who refuse to undergo certain cardiological tests. The main reasons identified for refusal to undergo stressful second-line tests such as cardiac MRI or a pharmacological challenge were related to accessibility and fear.
Consensus statements and clinical guidelines on the diagnosis and treatment of inherited CMs and channelopathies all emphasize the need to study the relatives of affected patients1,2 as this enables the detection of a considerable number of asymptomatic or oligosymptomatic patients who could benefit from lifestyle changes, medication, or other preventive strategies. According to some authors, over 60% of IHDs have been detected thanks to family screening.6,7
As our findings show, a significant proportion of individuals choose not to undergo recommended diagnostic procedures and treatments for different personal or logistic reasons.
Refusal to undergo family screening: opportunities for improvementSlightly over a quarter of the families studied chose not to participate in the IHD screening program. This means that only 1 patient—the proband—was evaluated in these families. Following evaluation of the family tree, all first-degree relatives are offered a screening appointment, but the necessary tests are not done. Refusal of screening was significantly more common in families with DCM (35%), possibly because of a lack of information or a lower awareness among healthcare providers about the hereditary nature of DCM (vs HCM and ACM). Participation in screening programs is higher in families with a history of SCD, possibly because of a greater sense of fear. Having moved to another region or country also accounts for a significant portion of reasons for nonparticipation in familial screening. This should not be an insurmountable problem in Spain, as these tests can be performed in another region and the Spanish Ministry of Health has a national system that provides coverage to patients at an accredited referral hospital or unit irrespective of their place of residence.
Diagnostic yield of family screeningOur findings confirm that active cardiac screening results in more families being diagnosed with IHD than questioning of probands only. The program we analyzed confirmed a diagnosis of IHD in 42% of families with a CM and in 30% of those with a channelopathy. These figures are consistent with previous reports.1.6 The net increase in diagnosis lies around 25%.
Refusal of second-line testsA considerable proportion (1 in every 5) of cardiac MRIs ordered were not performed. Regardless of the reasons, failure to perform these tests poses a considerable problem in terms of managing the resources of the IHD unit and the hospital. Administrative problems were listed as the reason for not attending an MRI appointment for over half the cases. Other reasons mentioned by the patients interviewed were fear, claustrophobia, and accessibility.
It has been proposed that it is difficult to determine the true prevalence of inherited channelopathies, as this requires asymptomatic relatives to undergo a pharmacological challenge, and over 40% refuse to do this. This high refusal rate could explain the low penetrance and varying prevalence rates reported for inherited channelopathies (e.g., 15%-37% for Brugada syndrome).6,8,9 We were pleased to observe that over 80% of patients advised to undergo a pharmacological challenges in our study agreed to do so. Several predictors of refusal were identified, including a history of arrhythmias (mainly atrial fibrillation) and others factors linked to a lack of prior information. Higher participation was associated with a positive genetic test result and a history of SCD in the family.
Our questioning of patients on why they did not undergo a pharmacological challenge also showed that a considerable percentage of individuals did not do so because they were afraid (46%) or did not consider it necessary (25%).
Refusal of recommended treatmentsOur findings show that only a minority of high-risk patients with a CM or a channelopathy refused to undergo IAD implantation (5%) or receive anticoagulation therapy (<3%). This rate is significantly lower than rates observed for the diagnostic tests, although it is not surprising as all the individuals in this sample had been diagnosed with an IHD. We did not detect any clear predictors of refusal to follow medical advice in terms of age, sex, or type of IHD. We did, however, find that a high percentage (almost 5%) of patients who refused ICD implantation died of SCD. By contrast, none of the patients who refused oral anticoagulation developed any complications as a result.
LimitationsOur findings may be affected by selection bias in relation to referral of patients considered to be at highest risk or to have more complex conditions to the IHD unit. In addition, participation by relatives in the screening program is voluntary and therefore the study of certain families may be incomplete. A family study was considered to have been performed when at least 2 relatives were evaluated. We also chose different subpopulations to meet each of our objectives, and this could complicate interpretation of our results. Our choice of second-line tests (cardiac MRI and pharmacological challenge) and treatments (IAD implantation and anticoagulation therapy) to meet objectives 2 and 3, respectively, was arbitrary. In addition, the small number of cases in certain subgroups and the relatively short follow-up time may have limited the statistical power of these analyses.
The design of the telephone survey did not allow for further investigation of the administrative problems mentioned by the patients who did not undergo second-line tests. Probable reasons include a lack of information on the test, scheduling delays, and problems establishing contact to arrange appointments.
ConclusionsOne in 4 families who were advised to undergo screening for a suspected IHD did not attend their appointment, and 1 in 5 individuals who did attend the screening appointment refused to undergo more sophisticated or stressful tests. We identified a number of independent predictors of refusal. Older patients were more likely to refuse sophisticated tests, and acceptance or refusal of tests was also influenced by female sex, a history of symptoms or arrhythmias, and genetic test result. Only a minority of high-risk patients refused ICD implantation or anticoagulation therapy.
FundingThis study was funded by a Nursing Training and Research Grant from the Spanish Association of Nursing in Cardiology. The research group is a member of the Biomedical Research Networking Center for Cardiovascular diseases (CIBERCV) and the Biomedical Research Networking Center for Rare Diseases (CIBERER). The Inherited Heart Disease and Sudden Cardiac Death is registered at the University of Murcia and the Instituto Murciano de Investigación Biosanitaria. The Inherited Heart Disease unit at Hospital Clínico Universitario Virgen de la Arrixaca is registered as a national referral unit (the CSUR system (Centros, Servicios y Unidades de Referencia en el Sistema de Salud [CSUR]) with Spanish Ministry of Health and is a member of the European Reference Network GUARD-Heart. M Sabater has a research contract with the Fundación para la Formación e Investigación Sanitarias de la Región de Murcia.
Conflicts of InterestThe authors do not have conflicts of interest in relation to this article.
- –
CMs and channelopathies are relatively common conditions associated with considerable morbidity and mortality.
- –
Even though active screening for IHD is recommended by clinical practice guidelines, most hospitals do not have these programs.
- –
We have analyzed the diagnostic yield of family cardiac screening and the difficulties associated with such a program.
- –
We have performed a detailed analysis of the reasons for refusal of diagnostic tests and treatment recommendations in patients with an IHD.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.06.014