To assess the efficacy of a comprehensive program of secondary prevention of cardiovascular disease in general practice.
MethodsA cluster randomized clinical trial was carried out in a regular general practice setting. Male and female patients aged under 86 years with a diagnosis of ischaemic heart disease, stroke or peripheral artery disease were recruited between January 2004 and May 2005. Study participants were seen at 42 health centres throughout the whole of Spain. The primary endpoint was the combination of all-cause mortality and hospital cardiovascular readmission at 3-year follow-up.
ResultsIn total, 1224 patients were recruited: 624 in the intervention group and 600 in the control group. The primary endpoint was observed in 29.9% (95% confidence interval [CI], 25.5%–34.8%) in the intervention group and 25.6% (22.3%–29.2%) in the control group (P=.15). At the end of follow-up, 8.5% (6.3%–11.3%) in the intervention group and 11% (7.4%–16%) in the control group were smokers (P=.07). The mean waist circumference of patients in the intervention and control groups was 100.44cm (95% CI, 98.97–101.91cm) and 102.58cm (95% CI, 100.96–104.21cm), respectively (P=.07). Overall, 20.9% (15.6%–27.7%) of patients in the intervention group and 29.6% (23.9%–36.1%) in the control group suffered from anxiety (P=.05), and 29.6% (22.4%–37.9%) in the intervention group and 41.4% (35.8%–47.3%) in the control group had depression (P=.02).
ConclusionsA comprehensive program of secondary prevention of cardiovascular disease in general practice was not effective in reducing cardiovascular morbidity and mortality. However, some factors associated with a healthy lifestyle were improved and anxiety and depression were reduced.
Keywords
The aim of secondary prevention strategies in patients with cardiovascular diseases is to reduce their risk of a new cardiovascular event and death and, therefore, to improve survival. It has been demonstrated that lifestyle changes, such as smoking cessation and a diet that modifies fatty acid profiles, lower cardiovascular morbidity and mortality in ischaemic patients and that physical exercise and cardiac rehabilitation after a myocardial infarction reduce the risk of cardiovascular deaths by 20%–25%. With respect to pharmacological treatment, we know that both prophylactic treatment and treatment designed to meet therapeutic objectives that target risk factors are beneficial.1,2
However, there are difficulties when it comes to incorporating the results of clinical trials into clinical practice. The comparison of the results of the EUROASPIRE I (1995–1996), EUROASPIRE II (1999–2000) and EUROASPIRE III (2006–2007) studies in patients with coronary disease show that the prevalence of risk factors continues to be high: smoking showed little variation (20.3%, 21.2%, and 18.2%), obesity (body mass index, BMI≥30) increased from 25.0% to 32.6% and 38.0%, and poorly controlled blood pressure (BP) (BP≥140/90mmHg) was scarcely modified (58.1%, 58.3%, and 60.9%). The only change was an important decrease in the prevalence of hypercholesterolaemia from 94.5% to 76.7% and 46.2%. As far as the use of prophylactic drugs is concerned, when the EUROASPIRE I and III studies3,4,5 were compared, anti-platelet agents were seen to increase from 80.8% to 93.2%; the use of beta blockers from 56% to 85.5%; all antihypertensive drugs from 84.5% to 96.8% and hypolipaemic agents from 32.2% to 88.8%. Although certain improvements have been seen in these three studies over the years, there is still a significant percentage of patients in whom the control of risk factors could be improved. Studies conducted in our centres also showed that 54% of patients with a history of myocardial infarction had hypercholesterolaemia, 41% were hypertensive, 11% were smokers and 19% were obese. In addition, there was patent underuse of prophylactic medication.6
Recently, the results of a primary care study in Ireland were published. In this trial an intensive intervention was applied and 18 months later there was a reduction in the number of readmissions, but other clinical benefits were not observed.7
In our setting different initiatives have been adopted to improve secondary prevention in coronary patients. In the PRESENTE8 study, the implementation of a simple intervention programme in patients who were admitted with myocardial infarction was evaluated and an improvement was observed after 6 months of follow-up of blood pressure, lipids, weight and other lifestyle-related parameters. The ICAR study, which was carried out in Catalonia, assessed the efficacy of an intensive programme designed to achieve secondary prevention of heart disease and implemented by GPs themselves. An improvement in the control of blood pressure and an increase in HDL-cholesterol levels were obtained, but no reduction in cardiovascular morbidity and mortality was observed.9,10
The fundamental aim of this study is to evaluate whether an intervention in patients who have already been diagnosed with cardiovascular disease (ischaemic heart disease, stroke and peripheral arterial disease) at the primary care level in different autonomous communities is effective in reducing readmission of patients with cardiovascular pathology and total mortality. The secondary objectives are to evaluate whether the intervention is effective in controlling cardiovascular risk factors and the use of prophylactic drugs.
MethodThe methodology for this study has already been described in previous articles.11,12 Briefly, this is a randomized, pragmatic, cluster (health centres) clinical trial conducted at the primary care level. The health centres were randomly assigned to either continue delivering the care they routinely provided to patients diagnosed with cardiovascular disease (control group or CG) or to organize a specific secondary prevention unit (intervention group or IG). This study was registered in the International Clinical Trial Register (ISRCT No. 18578323).
The randomization was centralized at the Fundació Institut Català de Farmacologia of the Hospital Vall d’Hebron in Barcelona and the randomization sequence was not revealed until the intervention was assigned (allocation concealment). As a way of restricting the random assignment sequence, the study population was stratified into autonomous communities in order to ensure that the two study groups were representative of each community. The study was open-label so nothing was concealed from the investigators with respect to the group to which they were assigned nor was there any concealment in the evaluation of the results. The study was actively monitored by internal and external auditors in order to guarantee the quality of the data.
Study SubjectsPatients from eight autonomous communities throughout Spain, who attended 42 health centres participated in the study.
Criteria for Participation in the StudyInclusion criteria: Men and women under 86 years of age and diagnosed between January 2004 and May 2005 with ischaemic cardiopathy (IC): acute myocardial infarction, unstable angina or acute coronary syndrome (confirmed by a hospital report), unstable angina confirmed by a diagnostic test (stress test and isotopic gammagraphy); cerebrovascular accident (CVA) verified by CT and/or a hospital report; and peripheral arterial disease (PAD) confirmed by ECHO Doppler or a positive ABI test.
Exclusion criteria: Patients with serious or terminal disease, who are bedbound and unstable (severe valvulopathies, post-infarction angina less than 28 days after acute myocardial infarction, severe ventricular arrhythmias in the last 6 months) or have suffered a subarachnoid haemorrhage and cardioembolic stroke as a result of valvulopathy which has already been diagnosed.
InterventionPatient visits were conducted in accordance with a protocol which was specific for each health centre group. All the IG and CG centres followed the protocols corresponding to their group. The intervention performed at the Health Centres assigned to the IG lasted 2 years and 9 months – one visit every 4 months – and was performed by previously trained nursing personnel. To be more specific, all the personnel who had to do the fieldwork attended a training workshop at the National Health School on the secondary prevention of cardiovascular disease in general and specific aspects of the study; afterwards there was another workshop, which was exclusively for the personnel who had to perform the intervention and covered more specific intervention aspects. The intervention consisted of: information about the disease, the introduction of lifestyle changes (promotion of suitable diets, physical exercise and giving up toxic habits), individualized intervention, depending on the risk factors for each patient and the therapeutic objectives targeted for each factor, and supervision of the treatment (both prophylactic treatment and treatment targeted at risk factors) which patients received. The personnel responsible for conducting the fieldwork had a guide on the intervention and the treatments consensually agreed by the research team, in which the different components of the intervention they were to perform, which were recorded in the data collection questionnaire, were protocolized. The patients assigned to the CG were seen at the beginning and at the end of the study period.
Primary and Secondary EndpointsData was collected about sociodemographic variables (age, sex, marital status, occupational status and education), toxic habits, clinical history (cardiovascular diseases prior to the patient being selected as a suitable study candidate, diabetes mellitus, hypertension, dyslipidaemia, chronic obstructive pulmonary disease, renal failure and psychiatric disease), complementary tests and analytical values (glycemic levels, total cholesterol, HDL- and LDL-cholesterol and triglycerides), as well as data concerning examinations (body mass index, abdominal perimeter, systolic and diastolic blood pressure) and prescribed pharmacological treatments. The primary endpoint was the combination of total mortality and hospital cardiovascular readmissions during the study period (ischaemic cardiopathy, cardiac failure, stroke and peripheral vascular disease). This information was collected by reviewing clinical records and from interviews with patients or their relatives at each of the follow-up visits in the case of the IG and at the last visit for the CG. The secondary endpoints were: health-related quality of life measured using the generic instrument known as the SF-36 health questionnaire, and anxiety and depression measured by means of the Goldberg Anxiety-depression Scale13 which had been translated and validated for its use in Spain.14
Data collection and analysis: Case report forms were designed for the initial visit, the follow-up visits and the final visit. Both the IG and CG patients were administered the SF-36 at the initial and final visits. With the intention of minimizing losses in terms of follow-up of IG patients who failed to attend scheduled visits, the latter were contacted in order to reschedule their visit.
Calculation of sample size: assuming an estimated annual principal event rate of 5% (15% at 3 years), an absolute reduction of 5% after 3 years, an intracluster correlation coefficient of 0.01, an average of 37 patients per cluster (health centre), an alpha error of 0.05 and a statistical power of 80%, it was calculated that 42 clusters would be needed, which would mean a total of 1554 patients. Assuming losses of 15% at 3 years it was estimated that a total sample of 1787 patients would be required.
Statistical AnalysisAll the analyses were performed taking into account the multicentre study design and its stratification according to autonomous communities. The baseline and results data were compared using the Chi-square test for the categorical variables, the Student t-test for continuous variables with a normal distribution and the Mann–Whitney test for variables which failed to show a normal distribution. Kaplan–Meier's curves were used to analyse survival and the survival curves of the study groups were compared by means of the log-rank test.
All the analyses of the efficacy of the intervention were performed by intention-to-treat analysis. All the IG patients were included in the analysis for their group, irrespective of the number of follow-up visits they attended. For the analysis of the primary variable IG patients were regarded as monitored subjects if data was available from their last or penultimate visit, in order to avoid losing patients for whom we had information during the entire follow-up period, except for the last visit. With respect to CG follow-up, subjects were contacted by telephone after 18 months of monitorization in order to verify vital data and again at the end of the study.
The data was analyzed using the STATA v 9.0 statistical program. Program routines designed for the analysis of complex samples, which enable study design, membership of clusters (health centres) and strata (autonomous communities) to be specified, and which adjust the results depending on the type of analysis, were used, variance being estimated by means of the Taylor first-order linearization technique. Statistical significance was established at levels below 5%.
ResultsOne thousand two hundred and twenty-four patients, 624 in the IG and 600 in the CG, were recruited. Their demographic and health characteristics, which showed no statistical differences between the two groups, can be seen in Table 1.
Table 1. Demographic and Health Characteristics of Study Groups (n=1224).
| Intervention group (n=624) | Control group (n=600) | |
| Age (years) | 65.73 (64.23-67.22) | 67.19 (65.95-68.43) |
| Sex (males) n (%) | 435 (69.7) | 426 (71.0) |
| Occupational status | ||
| Employed | 100 (16.0) | 87 (14.5) |
| Unemployed | 10 (1.6) | 15 (2.5) |
| On sick leave or invalidity | 98 (15.7) | 66 (11.0) |
| Retired | 338 (54.2) | 352 (58.7) |
| Other | 78 (12.5%) | 80 (13.3) |
| Educational status (n=1223) | ||
| Illiterate | 27 (4.3) | 26 (4.3) |
| No qualifications but able to read/ write | 157 (25.2) | 208 (34.7) |
| Primary level | 291 (46.7) | 239 (39.8) |
| Secondary level | 77 (12.4) | 81 (13.5) |
| Higher educational qualification | 43 (6.9) | 32 (5.3) |
| University diploma/degree | 28 (4.5) | 14 (2.3) |
| Smoker | 114 (18.3) | 94 (15.7) |
| Drinker | 42 (6.7) | 42 (7.0) |
| Patient obese/overweight | 412 (66.0) | 367 (61.2) |
| Goldberg Scale | ||
| Anxiety (n=1220) | 195 (31.3) | 207 (34.7) |
| Depression (n=1216) | 280 (45.2) | 277 (46.5) |
| Quality of Life (SF36) (n=1204) | ||
| Physical health | 40.40 (39.55-41.25]) | 40.22 (38.99-41.43) |
| Mental health * | 40.84 (39.77-41.91) | 42.29 (41.46-43.11) |
SF-36, SF-36 health questionnaire.
Data express mean (CI 95%) or n (%).
* P<.05.
The baseline clinical characteristics of the groups are shown in Table 2. The most common cardiovascular pathology in both groups was IC in 59.7% of cases, followed by CVA in 33.8% and PAD in 6.5%. Significant differences were not observed between the two groups with respect to their clinical characteristics.
Table 2. Baseline Clinical Characteristics of Study Groups (n=1224).
| Intervention group (n=624) | Control group (n=600) | |
| Cardiovascular pathology | ||
| Ischaemic heart disease | 377 (60.4%) | 354 (59.0%) |
| TIA or stroke | 203 (32.5%) | 211 (35.2%) |
| Peripheral vascular disease | 44 (7.1%) | 35 (5.8%) |
| Hypertension | 419 (67.2%) | 414 (69.0%) |
| Dyslipidemia | 379 (60.7%) | 332 (55.3%) |
| Diabetes | 188 (30.1%) | 192 (32.0%) |
| Previous medical history | ||
| Ischaemic heart disease | 174 (27.9%) | 156 (26.0%) |
| Heart failure | 45 (7.2%) | 48 (8.0%) |
| Cerebrovascular accident | 80 (12.8%) | 71 (11.8%) |
| Peripheral Arterial Disease | 42 (6.7%) | 52 (8.7%) |
| COPD | 48 (7.7%) | 48 (8.0%) |
| Renal failure | 21 (3.4%) | 28 (4.7%) |
| Psychiatric disease | 50 (8.0%) | 56 (9.3%) |
| Physical examination | ||
| Systolic blood pressure (mmHg) | 133.44 (130.67-136.21) | 134.58 (132.27-136.89) |
| Diastolic blood pressure (mmHg) | 76.05 (74.67-77.43) | 75.94 (74.93-76.95) |
| Body mass index (n=1222) | 28.72 (28.31-29.13) | 28.83 (28.26-29.39) |
| Abdominal circumference * (n=1220) | 100.09 (98.93-101.26) | 102.67 (100.79-104.55) |
| Analytical results (mg/dl) | ||
| Baseline glucose (n=1185) | 114.59 (111.59-117.61) | 116.34 (110.79-121.88) |
| Total cholesterol (n=1188) | 186.31 (182.14-190.48) | 185.28 (181.21-189.36) |
| LDL-cholesterol (n=1181) | 110.42 (107.20-113.64) | 112.06 (108.02-116.11) |
| HDL-cholesterol (n=1180) | 50.43 (47.72-53.14) | 49.29 (47.16-51.43) |
| Triglycerides (n=1164) | 128.71 (119.55-137.87) | 126.66 (119.07-134.26) |
Abbreviations: COPD, chronic obstructive pulmonary disease, HDL, high-density lipoproteins, LDL, low-density lipoproteins, TIA, transient ischaemic attack.
Data express mean (CI 95%) or n (%).
* P<.05.
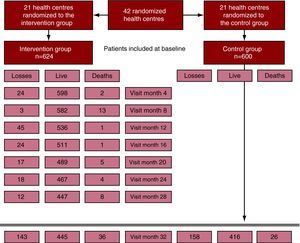
Figure 1 shows the flow chart for the study. Twenty-three percent of the patients were lost during follow-up, without there being any differences between the groups. The patients who were lost were not different to the patients who were followed up, either in terms of their demographic or clinical characteristics (results not shown). Amongst the lost patients, 67.1% in the IG group were overweight/obese compared to 80.4% in the CG (P=.009).
Figure 1. Study flow chart.
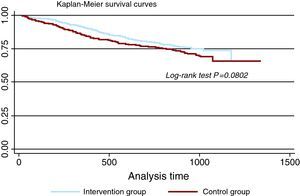
The follow-up results are shown in Table 3. Significant differences were not found between the two groups for combined episodes of death and readmission due to cardiovascular causes (29.9% vs 25.6%). For both groups the survival curves for the principal variable, which fail to show statistically significant differences, are shown in Figure 2.
Table 3. Final Results Classified According to Study Group.
| Patients (n=1224) | Intervention group (n=624) | Control group (n=600) | P value |
| Follow-up | |||
| Patients who were alive when contacted at last visit | 445 (71.3) | 416 (69.3) | .73 |
| Deaths | 36 (5.8) | 26 (4.3) | |
| Total number of follow-up patients | 481 (77.1) | 442 (73.7) | |
| Losses | 143 (22.9) | 158 (26.3) | |
| Death and/or cardiovascular hospital admissions | n=481 (77.1)144 (29.9) | n=442 (73.7)113 (25.6) | .15 |
| Follow up of surviving patients (n=861; 70.3%) | 445 (71.3) | 416 (69.3) | |
| Smoker (n=710) | 31 (8.5) | 38 (11.0) | .07 |
| Physical examination | |||
| Systolic blood pressure (mmHg) (n=844) | 132.88 (130.35-135.42) | 135.31 (132.65-137.98) | .15 |
| Diastolic blood pressure (mmHg) (n=846) | 74.93 (73.42-76.44) | 74.85 (73.63-76.06) | .93 |
| BP<140/90 in non-diabetics | 232 (52.1%) [47.0-57.2] | 205 (51.5%) [45.0-58.0] | .88 |
| BP<130/80 in diabetics (n=843) | |||
| Body mass index (n=824) | 28.73 (28.22-29.24) | 28.89 (28.34-29.43) | .69 |
| Abdominal circumference (cm) (n=833) | 100.44 (98.97-101.91) | 102.58 (100.96-104.21) | .07 |
| Analytical results (mg/dl) | |||
| Baseline glucose (n=833) | 113.99 (110.64-1187.35) | 110.27 (106.70-113.83) | .18 |
| Total cholesterol (n=838) | 177.46 (172.83-182.08) | 176.98 (172.32-181.64) | .89 |
| LDL-cholesterol (n=816) | 103.52 (98.84-108.19) | 105.07 (100.82-109.33) | .59 |
| HDL-cholesterol (n=824) | 51.22 (47.93-54.51) | 49.32 (47.34-51.29) | .40 |
| Triglycerides (n=827) | 126.43 (114.08-138.78) | 130.65 (121.42-139.87) | .59 |
| Goldberg Scale | |||
| Anxiety (n=753) | 85 (20.9%) | 103 (29.6%) | .05 |
| Depression (n=739) | 117 (29.6%) | 142 (41.4%) | .02 |
| Quality of Life (SF36) | |||
| Physical health (n=777) | 40.98 (40.19-41.76) | 40.94 (39.95-41.92) | .95 |
| Mental health (n=777) | 42.97 (41.90-44.04) | 44.29 (43.22-45.37) | .11 |
Abbreviations: BP, blood pressure; HDL, high-density lipoproteins; LDL, low-density lipoproteins; SF-36, SF-36 Health Questionnaire.
Data express mean (CI 95%) or n (%).
Figure 2. Survival graph for combined death and cardiovascular hospital readmission for each study group (patients who attended their final visit or died).
With respect to cardiovascular risk factors, the percentage of patients with well-controlled blood pressure (<140/90mmHg and <130/80mmHg in diabetics and subjects with chronic renal failure) was 52% in the IG and 51.5% in the CG (P=.88), the average glycemic level was 114 in the IG and 110 in the CG (P=.18), and the average IG LDL-c value was 103.5 and the CG equivalent was 105 (P=.59). The percentage of smokers was 8.5% in the IG and 11% in the CG (P=.07), and the mean abdominal perimeter was 100 in the IG and 102.5 in the CG (P=.07).
Significant differences were observed between the IG and the CG in terms of the percentage of patients with anxiety (20.9% vs 29.6%, P=.05) and depression (29.6% vs 41.4%, P=.02), their incidence being lower in the IG. Differences were not found in health-related quality of life between the two groups at the end of follow-up.
The treatments prescribed at baseline and at the end of the study for both groups are shown in Table 4. Significant differences were not detected for any drug, except in the use of aspirin, which increased in the IG while it decreased in the CG, and of antidepressants and anxiolytics (especially the former), which increased substantially in the IG.
Table 4. Standard Treatment for Study Groups.
| Intervention group | Control group | |
| Anti-platelet agents | ||
| Baseline visit | 531 (85.1) | 522 (87.2) |
| Final visit | 362 (81.4) | 338 (81.5) |
| Aspirin | ||
| Baseline visit | 405 (64.9) | 417 (69.6) |
| Final visit | 292 (65.6) | 277 (66.8) |
| Hypolipaemic agents | ||
| Baseline visit | 418 (66.9) | 400 (66.7) |
| Final visit | 327 (73.5) | 310 (74.7) |
| Statins | ||
| Baseline visit | 407 (65.2) | 394 (65.8) |
| Final visit | 321 (72.1) | 301 (72.5) |
| Beta blockers | ||
| Baseline visit | 279 (44.7) | 278 (46.6) |
| Final visit | 198 (44.5) | 203 (49.2) |
| ACE inhibitors | ||
| Baseline visit | 239 (38.4) | 242 (40.6) |
| Final visit | 166 (37.3) | 163 (39.5) |
| Angiotensin II antagonists | ||
| Baseline visit | 97 (15.6) | 110 (18.5) |
| Final visit | 101 (22.8) | 89 (21.5) |
| Anticoagulants | ||
| Baseline visit | 63 (10.1) | 64 (10.7) |
| Final visit | 60 (13.5) | 48 (11.6) |
| Antidiabetic drugs | ||
| Baseline visit | 157 (25.2) | 163 (27.3) |
| Final visit | 112 (25.2) | 114 (27.5) |
| Diuretics | ||
| Baseline visit | 92 (14.8) | 96 (16.1) |
| Final visit | 106 (23.8) | 82 (19.8) |
| Antidepressants | ||
| Baseline visit | 17 (2.7) | 17 (2.9) |
| Final visit | 34 (7.6) | 13 (3.1) |
| Anxiolytics | ||
| Baseline visit | 37 (5.9) | 38 (6.4) |
| Final visit | 40 (9.0) | 31 (7.5) |
ACE, angiotensin converting enzyme inhibitors.
Data express n (%).
In this study, which was conducted at the primary care level, we observed no differences in the main objective, which was to reduce morbidity and mortality as a result of cardiovascular diseases.
Although this was a clinical trial in which health centres were randomized to avoid the possible effects of cross-contamination between individuals, in the study patients were selected from clinical practice using inclusion criteria which were not very strict and, from this viewpoint, the trial could be regarded as pragmatic. Intentionally and so as not to distance ourselves from the intrinsic infrastructure of primary care teams, the intervention was administered by personnel from the health centres themselves so that patients probably experienced the intervention as part of their routine care.
This study has various limitations which need to be mentioned. The total recruited sample was eventually smaller than we had estimated in the sample calculation, which means there might not be enough statistical power to detect statistical differences. There could also be bias in the identification of events. Although there were no significant differences in losses between one group and the other, the IG patients attended visits regularly during the study and information was collected about possible events throughout the trial. In the CG, however, patients only attended an end-of-study visit (as well as receiving a phone call halfway through the study period) after three years of follow-up, so they could more easily have forgotten whether they had been admitted to hospital. Another study limitation is that cardiovascular events could not be cross-validated against hospital data or mortality records.
The lack of efficacy of the intervention could have a number of explanations. The first might be related to the clinical characteristics of patients lost during follow-up and the fact that these patients who were lost in the CG had a worse cardiovascular risk profile. To be specific, the percentage of overweight or obese patients was higher. Amongst those who completed follow-up, 65.7% of IG patients had a history of being overweight/obese as opposed to 54.3% of the CG patients (P<.001). A second explanation could be linked to an awareness on the part of the control group doctors that the clinical trial was being conducted, or the fact that they were more motivated with regard to cardiovascular prevention. Finally, there has been an increasing availability and implementation of guidelines in relation to the use of prophylactic drugs and the attainment of a series of therapeutic objectives. In these cases in which in routine practice intervention is fairly optimal, it is harder to find significant differences between control and intervention groups.
There are other aspects of the results which are worth highlighting. The cardiovascular risk profile at the end of the study was better in the IG than in the CG, in terms of abdominal obesity and tobacco, and prophylactic medication with anti-platelet agents and, specifically, the use of aspirin, which increases in the IG and decreases in the CG.
These aspects are clearly related to the intervention as such, given that the nursing personnel placed special emphasis on lifestyles and the importance of therapeutic compliance.
Studies which assess different strategies for improving secondary prevention results, using pragmatic randomized intervention designs at the primary care level, have been published with different results. One of these studies15 evaluated the effect of secondary prevention units run by nursing personnel, comparing them with routine care at GP surgeries. Improvements were seen in the use of anti-platelet agents, in blood pressure and lipid control, and in the performance of physical activity and following a healthy diet. However, changes with regard to giving up smoking were not observed. The same investigators subsequently published their follow-up findings after 4 years16 and they observed a reduction in mortality and coronary events when the two strategies were compared, although both authors comment that these results must be interpreted with caution, owing to the poor ability of the study to detect these differences and the borderline statistical significance values (P value borderline). Neither were significant differences in cardiovascular morbidity and mortality observed in the ICAR study9 after the implementation of an intensive programme that ensured patients were reminded to see their GP. In the SPHERE study7, which was conducted in Northern Ireland and in which health centres were also randomized, better control of risk factors in coronary disease patients was not observed, although there was a reduction in hospital admissions.
Another interesting result, which deserves to be highlighted, is the important reduction in the percentage of patients with depression in the IG compared to the CG, which was also associated with an important increase in antidepressant treatment in the IG. It has been demonstrated that depression is a predictor for the incidence of heart disease, and, moreover, it has a negative impact on prognosis, irrespective of other risk factors.17,18 According to the Goldberg scale, at baseline about 45% of the patients suffered from depression with no differences between the two groups, while at the end of the study 30% of the IG and 41% of the CG were depressed. As well as addressing classic risk factors and trying to meet the therapeutic targets set by guidelines, it is also important to take into account other factors which are linked to prognosis, such as depression, and to adopt the most appropriate therapeutic and non-therapeutic measures for these patients. When we analyzed the entire population (data not shown), 61% of the women and 39% of the men had been diagnosed with depression and 35% of the depressed patients suffered an event within 3 years, while 22% of the patients without depression suffered an event (P<.001). Future studies in the context of a clinical trial should explore the efficacy of detecting and treating depression and its treatment in the prognosis of patients who have suffered from cardiovascular disease.
ConclusionsA comprehensive secondary prevention of cardiovascular disease programme is not effective in reducing cardiovascular morbidity and mortality, but it is able to improve some aspects which are related to healthy habits and to reduce anxiety and depression in these patients.
FUNDINGProject coordinated and funded by the FIS (PI031421), Instituto de Salud Carlos III, Ministry of Health and Consumer Affairs. This project receives logistics support from the RedIAPP Cooperative Research Thematic Networks and RECAVA. Study registered in the International Standard Randomised Controlled Trial Register ISRCT No 18578323.
A.1. PREseAP Study InvestigatorsAragón: Ariño, Dolores (Main investigator); Abancens, Mercedes; Arroyo, Virginia; Miñana, Ana; Oliván, Bárbara; Reixa, Sol y Turón, José Mª.
Balearics: Borrás, Isabel (Main investigator); Benito, Ester; Brunet, Sofía; De la Cruz, Ana Belén; Escalas, Micaela; Escriche, Luis; Fiol, Francesca; Fullana, Francisca; Fullana Inmaculada; García, Basilio; Gastalver, Elvira; Gómez, Mª Pía; González, Mª del Carmen; Hernández, María; Mattei, Isabelle; Jaume, Maria de Lluch; Llobera, Joan; Mairata, Santiago; March, Sebastià; Marimón, Margarita; Mestre, Francisca; Miguélez, Angélica; Miralles, Jeroni; Mora, Brígida; Oliver, Margarita; Ortas, Silvia; Pascual, Catalina; Pieras, Josep; Rigo, Fernando; Rodríguez, Tomás; Ruíz, Isabel Mª; Salas, Isabel; Sancho, Salvadora; Useros, Victoria.
Castilla and Leon (1): Rodrigo, Mª Pilar (Main investigator); Bernardos, Magdalena; Del Teso, José Mª; Del Valle, Mª Antonia; Granja, Yolanda; Marchessi, Mª Jesús; Redondo, Jesús.
Castilla and Leon (2): González, Mª Luisa (Main investigator); Alvárez, Violeta; De Juan, Noemí; Gonzalo, Mª Visitación; Higuera, Evelio; Luis, Encarna; Martínez, Itziar; Pereda, Mª José.
Catalonia (1): Brotons, Carlos (Main investigator); Closas, Vanesa; Corral Rosario; García, David; Gràcia, Lluis; Gutiérrez, Silvia; Iruela, Antoni; Martínez, Mireia; Moral, Irene; Morató, Mª Dolors; Palau, Antoni; Payan, Miriam; Pérez, José; Rayó, Elisabet; Soriano, Núria; Vila, Francesc; Yrla, Rosa.
Catalonia (2): Pepió, Josep Mª (Main investigator); Aguilar, Carina; Albero, Jordi; Arasa, Concepción; Arasa, Mª José; Beguer, Nuria; Bertomeu, María; Carcelle, Josep P; Checa, Encarnación; Ciurana, Emilio; Ciurana, Maria Riera; Clua, Josep Lluis; Curto, Claudia; Dalmau, Mª Rosa; Daniel, Jordi; Fatsini, Mª Merçé; Ferré, Inmaculada; García, Gracia; Grau, Araceli; Guasch, Joan Lluís; Juan, Roland; Llor, Josep Lluís; Marín, Judit; Monclus, Josep Felip; Pons, Jaime; Ramos, Josep J; Santigosa, Joan.
Extremadura: Buitrago, Francisco (Main investigator); Cañón, Lourdes; Casquero, Mª Pilar; Cruces, Eloísa; Díaz, Natalio; Navarro Elisabet; Nogales, Ramón; Serrano, Mª Victoria; Velasco, Carmen.
Madrid: Kloppe, Pilar (Main investigator); Auñón, Angela; Canellas, Mercedes; Costa, Pilar; Fernández, Carmen; Garro, Mª Angeles; Gómez, Rosario; Herradura, Pura; Jimeno, Milagros; Pastor, Ana; Piñero, Mª José; Rapp, Pilar; Segura, Roberto; Sierra, Eva.
Basque Country: Rodríguez, Ana Isabel (Main investigator); Benavides, Raquel; Celma, Dolores; Fuentes, Conchi; Ortueta, Pedro.
Valencia: Orozco, Domingo (Main investigator); Carratalá, Concha; Fluixà, Carlos; Galán, José; Galinsoga, Mª del Carmen; Gil, Vicente; Huertas, Adela; López, Mª Isabel; Maiques, Antonio; Marco, Rocío; Martínez, Nieves; Mas, Francisco; Navarro, Jorge; Navarro, Mercedes; Payá, José Jorge; Pereira, Avelino; Prado, Pilar; Prieto, Isabel; Quirce, Fernando; Richart, Miguel; Séller, Mª Jesús; Sevilla, Fernando; Sierra, Eva; Siurana, Milagros; Soler, José Manuel; Terol, Cecilia.
Advisory Committee: Diògene, Eduard; Del Rio, Alfonso; Gil, Antonio; Gordillo, Ma Victoria; Muñoz, Miguel Angel; Vidal, Xavier; Villar, Fernando.
Received 9 March 2010
Accepted 19 July 2010
Corresponding author Unidad de Investigación, EAP Sardenya-IIB Sant Pau, Sardenya, 466, 08025 Barcelona, Spain. cbrotons@eapsardenya.cat