Keywords
INTRODUCTION
Fifteen years after being introduced,1 radial access for cardiac catheterization is being adopted by a growing number of interventional cardiologists.2,3 In comparison with femoral access, the radial route is safer and reduces patient discomfort,4 especially in certain subgroups (older and/or obese patients, those undergoing an intervention for a myocardial infarction, etc).5,6 The radial artery is a thick-walled vessel composed mainly of smooth muscle cells arranged in concentric layers. This marked muscular component of the artery, together with the high density of alpha-1 receptors, makes this vessel especially susceptible to spasms.7 The occurrence of radial artery spasm offsets the advantages of this route of access, increasing the degree of patient discomfort and reducing the chances of a successful catheterization. Even in centers where there is extensive experience with the radial route, radial spasm occurs in 15% to 30% of the procedures.8
Spasm of the radial artery employed as a coronary artery bypass graft and the measures taken to prevent it have been extensively studied.9,10 However, few reports have focused on the analysis of factors involved in radial artery spasm when this artery is used as the route of access in cardiac catheterization.8,11
The purpose of this work was to study radial artery spasm in transradial cardiac catheterization and, specifically, to identify factors associated with its development and analyze its consequences following the procedure.
PATIENTS AND METHOD
Patients
Of 659 patients in whom transradial catheterization was attempted between November 2003 and April 2004, 637 in whom a successful and atraumatic radial artery cannulation was achieved were consecutively enrolled in the study.
Procedure
The use of the radial approach has been extensively described, and the radial artery is the route of choice in our center. For this study, the operator chose the radial access and either the left or right side depending on the pulse quality and the results of a modified Allen's test. The patients were systematically sedated with 10 mg of oral diazepam one hour before the procedure. A topical anesthetic cream (lidocaine, AstraZeneca®) was applied around the radial puncture site in a group of randomly selected patients. Once in the laboratory, all patients received local anesthesia with 1 mL of mepivacaine, after which the radial artery was cannulated using a 20-gauge needle (Vigon®). An introducer (Maximun, St. Jude Medical®) was employed to administer 5000 units of heparin and a spasmolytic agent (2.5 mg of verapamil or phentolamine) dissolved in 20 mL of saline solution.
The diameter of the introducer and the diameter and curve of the catheter were chosen according to the criteria of the operator. Initially, all the procedures were planned as diagnostic studies. Once they had been completed, a decision was made as to whether or not to perform an ad hoc percutaneous coronary intervention.
Evaluation of the Radial Spasm
The operator assessed the radial spasm on the basis of a questionnaire addressing the following five signs: persistent forearm pain, pain response on catheter manipulation, pain response to introducer withdrawal, difficult catheter manipulation after being "trapped" by the radial artery with considerable resistance on withdrawal of the introducer. Radial spasm was considered to be indicated by the presence of at least 2 of these 5 signs or by the presence of just 1 when the operator considered it necessary to administer a second dose of the spasmolytic agent.
In addition to the presence of radial spasm, the following procedure-related variables were analyzed:
- Prior preparation with the topical anesthetic cream.
- Pain intensity during radial artery cannulation (radial artery puncture and insertion of the introducer) as perceived by the patient. Pain was scored on a scale of 0 to 3 (0, absence of pain; 1, mild pain; 2, moderate pain; and 3, severe pain). In each case, the patient was asked to differentiate between the pain experienced during radial artery cannulation and that felt while catheterized (radial artery spasm).
- Cannulation of the right or left radial artery.
- Type of procedure (diagnostic or diagnostic followed by an ad hoc intervention.
- Length of the procedure, defined as the time elapsed between insertion of the introducer and its withdrawal.
- Tortuosity of the subclavian artery. The subclavian artery was considered to be tortuous when, in order to get past the tortuous section, certain maneuvers were required: sustained deep inspiration, head turned toward the contralateral side and substitution of the standard Teflon-coated guidewire by a Terumo guidewire. A retroesophageal subclavian artery was considered to be a tortuosity.
- Total number of catheters employed (3 or less, or more than 3).
- Initial and maximum diameters of the catheters employed, measured according to the French system.
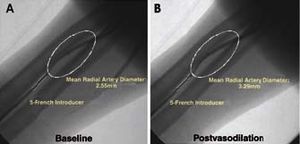
Angiographic Evaluation of the Radial Artery (Figure 1)
Figure 1. Angiographic study of the radial artery under baseline conditions (A) and 2 minutes after the intraradial administration of the vasodilator (B).
The diameter of the radial artery was determined by angiography. For this purpose, the contrast agent was injected via the introducer before and 2 minutes after the administration of the vasodilator.
A 30 to 40-mm long segment of the artery was selected for the determination of the mean diameter of the vessel using a computer-assisted quantification method (QUANTCOR, Siemens®). The distal portion of the introducer was used as a reference. With the aid of precise anatomical markers, the selected segment was measured under baseline conditions and after vasodilator administration. In addition to the mean diameters, we calculated the radial vasodilatory capacity using the following equation: postvasodilation diameter--baseline diameter/baseline diameter.
All measurements were performed by the same operator.
Radial angiography also enabled us to detect the anatomic anomalies of this vessel. All these findings were systematically collected.
Follow-up
All the patients enrolled in the study were asked to return within 1 month of the procedure for assessment of the radial access. During this examination, different operators, unfamiliar with the events of the original procedure, assessed the presence (at any time) of the following:
- Pain. The patient was asked about the presence of pain in the area of the puncture on the days after the procedure. The level of pain was again assessed according to the same 4-point scale (0, absence of pain; 1, mild pain; 2, moderate pain; and 3, severe pain).
- Hematoma. Hematomas measuring more than 3 cm, even if superficial, were recorded.
- Radial artery patency. The patency of the radial artery was assessed using pulse oxymetry and plethysmography in thumb during a modified Allen's test. This method is employed in the analysis of the patency of the vascular structures of the palmar arch12 and offers the advantages over ultrasound of its greater availability and lower variability. Three degrees of radial patency were recorded: patent radial artery (normal pulse wave and saturation), partially occluded (undetectable pulse wave on cubital compression but saturation mantained) and occluded (undetectable pulse wave and saturation). Patients with radial occlusion were asked to return for further reassessment. Those who were unable to return for follow-up were interviewed by telephone with regard to pain intensity during the postoperative period and the presence of hematoma.
Statistical Study
The statistical analysis was performed with the SPSS software package (version 10.0) for Windows. We used a hybrid model (cross-sectional and descriptive cohort) to analyze the variables associated with the development of spasm during the procedure and evaluate its impact during follow-up. Continuous variables were expressed as the mean, plus or minus the standard deviation, and categorical data as percentages. After dividing the study population into 2 groups according to whether or not they had experienced radial spasm, we used Student's t test to compare the values for each continuous variable, whereas the *2 test was applied to the categorical variables. The variables for which univariate analysis revealed statistically significant differences (age, sex, number of catheters employed [3 or less vs more than 3], intensity of pain during radial cannulation [mild or no pain vs moderate or severe pain], duration of the procedure, vasodilation cocktail [phentolamine vs verapamil], radial artery anatomy and diameter of the radial artery following vasodilation) were studied using step-wise logistic regression analysis to determine the strength of association (odds ratio [OR]) with radial spasm. The variable "type of procedure" was not included in this model as it was strongly associated with the variable "number of catheters employed." The goodness of fit of the logistic model was analyzed by means of the Hosmer-Lemeshow test.
RESULTS
The population consisted of 637 patients, 127 (20.2%) of whom experienced radial spasm during the procedure. The route of access was changed in 25 patients (3.9%), a measure that was due to radial spasm in 13 cases (2% of the overall group).
The clinical characteristics of the patient population, grouped according to whether or not they had developed radial spasm, are shown in Table 1. The patients with vasospasm were significantly younger (61.0±14.0 years vs 63.5±11.3 years; P=.03) and included a higher relative percentage of women.
Features Differentiating Patients Who Developed Radial Spasm From Those Who Did Not (Table 2)
In the majority of cases, the procedure was performed by the right radial artery (88.5%) and was begun with a 5 French catheter (66.4%). Neither the access side (right or left radial artery) nor the initial or maximum catheter diameter (4 to 6 French) was associated with the development of radial spasm. Patients who received phentolamine (48%) had a significantly higher incidence of vasospasm than those who received verapamil (23.2% vs 16.9%, respectively; P=.04).
The intensity of the pain during radial artery cannulation, as perceived by the patient, was significantly related to the occurrence of radial spasm. Overall, the majority of the patients reported mild pain during radial cannulation; however, the pain during the procedure was perceived to be moderate or severe by 23.8% of the patients in the vasospasm group, versus 8.5% of those in the group without vasospasm (P<.001). On the other hand, the combination of the procedure with an ad hoc intervention (as compared to merely diagnostic catheterization) and the use of more than three catheters during the procedure were also significantly related to radial spasm (P<.001 in both cases).
Angiographic Features of the Radial Artery and the Development of Vasospasm
The mean baseline diameter of the radial artery in our patient population was 2.20±0.55 mm (range, 0.65 to 4.30 mm) and increased to 2.46±0.54 mm (range, 0.65 to 4.43 mm) after administration of the vasodilator. Patients with vasospasm during the catheterization had significantly smaller mean radial artery diameters both at baseline (2.03±0.48 mm vs 2.24±0.56 mm; P<.001) and after vasodilator administration (2.27±0.46 mm vs 2.51±0.54 mm; P<.001). There were no significant differences in the vasodilatory capacity of the radial artery when the patients who developed vasospasm were compared with those who did not (13.0±15.1 vs 13.4±16.0, respectively).
On the other hand, the presence of a radial artery anatomical anomaly was also associated with the development of radial spasm (P<.001). There were 37 cases of anomalous radial arteries, corresponding to 5.8% of the study population. Vasospasm presented in 19 of these patients (51.4%), versus a rate of 18.0% among patients with normal radial artery anatomy. The types of anomalies detected and the relative incidence of each of them appear in Table 3.
Independent Predictors of Radial Spasm (Figure 2)
Figure 2. Independent predictors of radial spasm during cardiac catheterization.
Logistic regression analysis showed that a radial artery anatomical anomaly was the independent variable having the strongest association with spasm (OR=5.1; 95% confidence interval [CI], 2.2-11.4; P<.001).
The other variables independently associated with radial spasm were the use of more than 3 catheters during the procedure, moderate-to-severe pain during radial cannulation and the use of phentolamine as the spasmolytic agent. Finally, the diameter of the radial artery after vasodilation was independently and inversely associated with the development of spasm.
Patient Follow-up: Impact of Radial Spasm Occurring During Catheterization (Figure 3)
Figure 3. Consequences of radial spasm during follow-up (20±18 days postprocedure). Only forearm pain on the days following the procedure was significantly more intense in the patients who had experienced vasospasm. There were no significant differences in the incidence of hematoma or radial artery occlusion.
In all, 94.8% of the study population (604 patients) returned for a follow-up visit 20±18 days after the performance of the procedure. In the majority of the cases (88.9%), follow-up (case history, physical examination and assessment of radial artery patency by plethysmography, and pulse oximetry) was carried out in a clinical visit.
Most of the patients reported mild pain or no pain at all in the forearm during the follow-up period (93.3% of the 599 patients who responded). Pain was moderate or severe in 40 patients (6.7%). The intensity of the pain in the area of the puncture was related to the occurrence of radial spasm during the procedure (P=.003): 12.4% of the patients who experienced vasospasm complained of moderate or severe pain during follow-up, versus 5.3% of those in whom vasospasm did not occur.
Hematoma was present in 64 of the 600 patients evaluated (10.7%). The hematoma was superficial in all but one case, in which it was severe owing to the perforation of the radial artery during the procedure; eventually a satisfactory outcome was achieved with conservative treatment. There were no significant differences between patients with and without vasospasm in terms of the presence of hematoma (10.7% vs 10.6%, respectively).
Finally, the degree of patency of the radial artery was assessed in 529 patients (83.0% of the study population). In 93.4% of the cases, the artery was fully patent, in 21 cases (4.0%) it was considered to be partially occluded and in 14 patients (2.6) it was occluded. There was no significant association between radial spasm and patency during follow-up, although there was a higher percentage of radial artery occlusion among the patients in whom vasospasm had occurred (4.5% vs 2.2%). All the patients with occluded radial artery were asymptomatic with the exception of one, who complained of claudication of his hand after prolonged efforts.
The patients with occluded radial artery were reassessed 116±47 days after the catheterization (with the exception of one, who died during follow-up). The artery remained occluded in only 2 cases (0.3% of those evaluated), both involving patients who had experienced radial spasm.
DISCUSSION
The vasospastic potential of the radial artery is intermediate between that of the splanchnic arteries (the hypogastric and gastroepiploic arteries, with greater predisposition toward spasm) and that of the somatic arteries (internal mammary artery). The marked muscle mass in the radial artery wall, which is greater than that of the other arteries, and its high density in alpha-adrenergic receptors explain its proclivity to spasm.9,10 Although the incidence of radial spasm during catheterization depends on the experience of the operator,13 this complication occurs in 15% to 30% of the procedures performed even at the most highly experienced centers.8 The development of radial vasospasm offsets the advantages of this access since the trapping of the catheter by the artery generates pain and makes its manipulation difficult or even impossible. Moreover, radial spasm can produce other serious complications, such as the perforation of the vessel, and even there have been rep orts of its traumatic section when an attempt was made to withdraw the introducer.14
Radial Spasm in Our Patient Population
In our study, the incidence of vasospasm was 20%. The first problem to face in a study of this type is the lack of a clear definition of radial spasm during transradial catheterization. Only 1 objective method for quantifying the degree of spasm has been described. It consists of a mechanism that measures the force required to withdraw a 23-cm-long introducer from the radial artery.15 This manner of determining the degree of spasm is not applicable in our setting, where the generalized trend is to employ shorter radial introducers. For this reason, we opted for creating our own scale to evaluate its impact on patient discomfort and technical difficulty for the surgeon.
Radial Artery Characteristics Predictive of Vasospasm
The variable that showed the strongest association with radial spasm was the existence of an anatomical anomaly, a circumstance that resulted in a 5-fold increase in the likelihood of spasm. In our patient population, the incidence of these anomalies was 5.8%, a frequency that was similar to that reported by other authors.16 The most common radial artery anomaly in our study group was the ectopic origin of the artery, generally in the axillary brachial plexus. This marked predisposition of anomalous radial arteries to present spasm (which occurred in somewhat more than half of the cases of anatomical anomaly) may be due to the greater manipulation required for its cannulation, although the possibility that the anatomical anomaly results in an underlying spasmogenic functional anomaly can not be ruled out.
The size of the radial artery was also independently associated with spasm. It has been reported that the utilization of larger devices in radial arteries having a small diameter provokes greater vascular injury.17 In our patient population, the mean radial artery diameter after vasodilation was 2.46 mm, similar to that reported for other series in which the intraluminal diameter was measured using ultrasound.18 The diameter of the radial artery was significantly smaller in the patients who experienced vasospasm. This relationship between a smaller radial size and a greater propensity for spasm highlights the need to assess the size of the artery, on the basis of the pulse quality, in the initial examination, following the evaluation of the Allen test. This would enable the operator to choose the appropriate material in each case.
Procedural Variables Associated With Radial Artery Spasm
The most complex procedures, particularly those requiring the use of more than 3 catheters (difficult coronary catheterization, percutaneous interventions), are related to the development of spasm; this could serve as an argument for those operators who consider that, as a precaution against a complicated catheterization, the femoral approach is preferable.
The intensity of the pain perceived by the patient at the start of radial cannulation was associated with vasospasm; the incidence of spasm was greater among those patients who reported more severe pain. The increase in circulating catecholamines as a consequence of the pain, or even a high degree of precatheterization anxiety, might explain the greater incidence of vasospasm due to alpha-adrenergic stimulation.7 Thus, the importance of the proper sedation of the patient prior to the procedure and the need to create an atmosphere of tranquility during the catheterization should be stressed.
Finally, the composition of the spasmolytic agent, specifically the administration of phentolamine, was also associated with the development of radial spasm. Although this alpha-adrenergic blocker has been shown to be effective in preventing radial artery spasm when this vessel is employed as a coronary graft,19 in our series it proved to be inferior to verapamil as a spasmolytic agent.
Consequences of Radial Spasm in Patient Follow-up
The occurrence of radial spasm during cardiac catheterization resulted in more severe pain in the puncture zone over the subsequent days. One of the advantages of radial access is the absence of nerve structures adjacent to the artery, a circumstance that reduces the risk of neurogenic complications. However, the development of chronic regional pain syndrome following the transradial procedure has been reported.20 In our series, all the cases of moderate and severe pain were limited to the first or second week after the intervention, and they usually involved a distribution similar to the brachioradial axis, with the pain reaching the elbow or even the shoulder. Patients with severe pain were treated empirically with topical nonsteroidal anti-inflammatory agents.
Although radial occlusion is generally asymptomatic, it is the most widely feared complication of the transradial procedure. It has been related to the severity of the lesion suffered by the artery during the procedure;17 thus, radial spasm may be associated with subsequent occlusion. In our population, we found a rate of radial occlusion during the first month after the procedure of 2.6%, similar to that reported for other studies.21 The incidence of radial occlusion among the patients who experienced spasm was higher than that of the group without this problem, although we observed no significant relationship between spasm and radial artery occlusion. The latter is largely a reversible process,18 perhaps because of the fact that the principal cause is the negative remodeling of the vessel after the stretching it undergoes during cannulation; intimal hyperplasia and radial thrombosis are secondary mechanisms. In our series, radial occlusion persisted in only 2 patients (0.3% of the study population).
Limitations
The major limitation to this study was the evaluation of radial spasms by means of an unvalidated, self-administered questionnaire; however, our aim was to identify the different events that can be provoked by radial spasm during catheterization. The criteria according to which only those patients in whom radial cannulation was done successfully and atraumatically were included could result in the underestimation of the number of cases in which the attempt to perform the procedure failed, although it is known that the majority of the failed attempts are due to a hypoplastic or tortuous radial artery.16 Finally, another limitation is the need to distinguish between the pain owing to radial artery cannulation and that produced during the procedure by vasospasm: when asking the patients, the operators were careful to pose questions that would make it easier to make the distinction between them.
CONCLUSIONS
Radial spasm occurs in one of every five patients undergoing transradial cardiac catheterization, although it makes it necessary to continue the procedure through a different access route in only 2%. Certain anatomical features of the radial artery and variables related to the procedure determine the development of vasospasm. An anomalous radial artery is the major predisposing factor, while a smaller diameter is also associated with radial spasm. Other predisposing variables over which we can have a greater control include the type of vasodilator employed as a spasmolytic, the intensity of the pain experienced by the patient during radial cannulation and the complexity of the procedure. Aside from patient age, we have found no other clinical variable associated with radial artery spasm. The consequences of spasm are reduced to more severe forearm pain on the days following the procedure and, in 2.6% of the patients, radial artery occlusion within 1 month. We observed no significant relationship between radial artery spasm and subsequent occlusion of the vessel.
Correspondence: Dr. R.J. Ruiz-Salmerón.
Departamento de Hemodinámica y Cardiología Intervencionista. Hospital Clínic.
Villarroel, 170. 08036 Barcelona. España.
E-mail: rjruiz@clinic.ub.es
Received November 18, 2004.
Accepted for publication February 7, 2005.