The aim of the study was to assess and measure health-related quality of life (HRQoL) changes in patients with typical atrial flutter following catheter ablation. The outcome was standardized and normalized to the Spanish population adjusted by age and sex.
MethodsNinety-five consecutive patients who had undergone cavotricuspid isthmus ablation were included. The SF-36 questionnaire was self-administered before the procedure and at 1-year follow-up. We used the effect size and the standardized response mean as measures of responsiveness to quantify the change in HRQoL and the minimum clinically important difference to assess the smallest difference in score that patients perceived as beneficial.
ResultsOf the 95 patients initially included, 88 completed the 1-year follow-up. We observed a large improvement (effect size ≥0.8) on the physical functioning, role-physical, general health, and vitality scales and on the physical component summary. We detected a moderate improvement (effect size ≥0.5) on the role-emotional, social functioning, and mental health scales and on the mental component summary. On all scales except bodily pain and social activity, the improvement was clinically perceived by patients.
ConclusionsA clinically significant improvement in HRQoL measures was found in patients with typical atrial flutter who underwent cavotricuspid isthmus catheter ablation.
Keywords
Typical atrial flutter (AFl) is an arrhythmia that originates in the right atrium through a mechanism involving a reentrant circuit around the tricuspid valve that involves the cavotricuspid isthmus (CTI). It is responsible for 75% to 90% of macroreentrant atrial tachycardia.1CTI ablation is a first-line treatment option for typical recurrent AFl, especially in cases with poor clinical tolerance. It is also indicated in patients in whom AFl develops following pharmacological treatment of atrial fibrillation (AFib) with class I or III drugs.2, 3, 4
Studies have shown an improvement in symptoms and quality of life following CTI ablation.5, 6, 7, 8 Nevertheless, those studies used the American population as a reference and did not adjust for age and sex, factors that have a significant effect on quality of life.
Furthermore, recent years have seen rapid advances in the assessment of health status, with the incorporation of new quality-of-life measures. Minimum clinically important difference (MCID) and minimum detectable change are relevant concepts for the determination of changes in quality of life following an intervention (responsiveness) and are currently an important area of research.
The aim of this study was to evaluate quality-of-life changes in a cohort of unselected, consecutive patients with CTI-dependent AFl referred for radiofrequency catheter ablation. Data were standardized and normalized for the Spanish population, adjusted for age and sex. In addition, we quantified the effect size obtained and expressed the results in terms of MCID to determine whether the improvement in quality of life was sufficient for patients to perceive a significant benefit in terms of symptoms.
Methods Study PopulationIn total, 95 consecutive patients referred to the cardiac eletrophysiology laboratory between January 2003 and March 2005 were included in the study. All patients met the following inclusion criteria: a) at least 18years of age; b) 1 or more episodes of AFl documented by 12-lead electrocardiogram in the last 6 months; c) a history of isolated AFl or predominant AFl with concomitant AFib or AFl following treatment with type I or type III antiarrhythmic drugs for the prevention of AFib; and d) electrophysiologic confirmation of CTI-dependent AFl or CTI permeability if the ablation procedure is performed in sinus rhythm. In that case, the requirement was that there be a common typical electrocardiogram of the clinical episode.
The following exclusion criteria were applied: a) presence of non-CTI-dependent AFl; b) heart surgery or cardiac interventional procedure (coronary angioplasty or pacemaker implantation) in the last 30days; c) use of an implantable cardioverter defibrillator; d) life expectancy <1 year; and e) inability to complete a quality-of-life questionnaire.
A patient was considered to have a prevalent disease, such as hypertension, diabetes mellitus, hyperlipidemia, pulmonary disease, or arthritis, if the diagnosis had been made by a physician. Obesity was defined as a body mass index >30.
DefinitionsTypical flutter: Morphology of the flutter waves in the lower leads characterized by a slow descending slope, followed by a sharp descending slope and a sharp ascending slope, and ending with a low-amplitude positive component that leads into the gradual descending segment of the following flutter wave. The flutter waves are positive in V1 and negative in V6.
Atypical flutter: Wide, positive flutter waves with notches in the lower leads with a negative component that precedes the positive component, and with wide negative waves in V1.
Ablation ProcedureThe procedure was undertaken following at least 6h of fasting. Oral anticoagulation was suspended 2days before the electrophysiology study and treatment with low-molecular-weight heparin was initiated if the international normalized ratio was <1.5. A standard quadripolar catheter (Usci-Bard Inc.) was used to map the His bundle region, a decapolar catheter (Usci-Bard Inc.) to map the coronary sinus, and a duodecapolar Halo XP catheter (Cordis-Webster Inc.) to map the activation of the anterolateral wall of the right atrium. Radiofrequency energy was applied for a period of 60s at each point. CTI dependency was confirmed by entrainment when the rhythm at the beginning of the electrophysiology study was AFl or when this was induced in the laboratory. If the patient was in sinus rhythm, bidirectional CTI permeability was confirmed prior to ablation. The aim of the procedure was to achieve bidirectional CTI conduction block.9, 10 Bidirectional block was defined through the activation sequence of the electrograms in the right atrium, the bundle of His, and the coronary sinus stimulating at a cycle length of 600ms from the coronary sinus and from the lower lateral wall of the right atrium. The persistence of bidirectional block was confirmed 20min after completion of the procedure.
QuestionnaireThe SF-36 questionnaire11 was used as an instrument to measure health-related quality of life (HRQoL). The questionnaire was completed by patients prior to ablation and at 1-year follow-up. The questionnaire comprises 8 scales or dimensions that are transformed into scores of between 0 and 100, such that the higher the score the better the health status of the patient.11 An additional item, known as the self-reported health transition, measures the change in health status over a period of 1 year, although it is not included in the analysis alongside the 8 dimensions (Table 1). Each of the scales was standardized and normalized for the Spanish population adjusted for age and sex, such that the reference values have a mean (standard deviation [SD]) of 50 (10). Slight changes are defined as differences of more than 4 points, moderate changes as differences of more than 6 points, and large changes as differences of more than 8 points.12, 13, 14
Table 1. Scales on the Short Form-36 Questionnaire.
| Scales or Dimensions | No. of Items | Summary of Content |
| Physical functioning | 10 | Extent to which health limits physical activities such as self-care, walking, climbing stairs, bending, lifting or carrying weights, and moderate or intense effort |
| Role-physical | 4 | Extent to which physical health interferes with work and other daily activities, such as lower than desired productivity, limitation of the type of activities performed, or difficulty in performing activities |
| Bodily pain | 2 | Pain intensity and its effect on usual work both at home and outside of the home |
| General health perceptions | 5 | Personal assessment of health including current health, future health perspectives, and resistance to illness |
| Vitality | 4 | Feeling of energy and vitality versus feeling of tiredness and exhaustion |
| Social functioning | 2 | Extent to which problems with physical or emotional health interfere in usual social life |
| Role-emotional | 3 | Degree to which emotional problems interfere with work or other daily activities, such as a reduction in the time dedicated to those activities, lower than desired productivity, and reduced care and attention to work |
| Mental health | 5 | General mental health, including depression, anxiety, behavioral control, and general well-being |
| Health transition | 1 | Assessment of general health compared with 1 year previously |
Through a combination of the scores on each dimension, the questionnaire allows 2 summary scores to be calculated: the physical component summary (PCS) and the mental component summary (MCS).15
To quantify the response obtained, the effect size (ES) was measured along with the standardized response mean (SRM) for each scale on the questionnaire. The ES is calculated as the difference between the mean values obtained for each dimension at baseline and follow-up divided by the SD of the baseline group. The SRM is calculated as the difference of the mean values on each dimension at baseline and follow-up divided by the SD of the difference between baseline and follow-up. Positive values indicate improvement in the number of times that the result contains the SD of the baseline group (ES) or the SD of the difference between the groups (SRM) for each dimension.16 The ES does not have units and is quantified as follows: a) large effect, ≥0.8; b) moderate effect, 0.50-0.79; c) small effect, 0.20-0.49; and d) very small effect, (0.00-0.19).
Another way to quantify the ES is to compare it with the minimum important difference (MID).17, 18 This is defined as the smallest measurable difference in health status that corresponds to an important difference in patient symptoms. It is estimated based on determination of the standard error of the mean (SEM). The SEM is defined as the standard error in the observed result that obscures the true result, and its value is independent of the sample.18 This last property makes a good estimate of individual changes in an indicator of HRQoL.
SDx: baseline SD of each dimension.
rxx: intraclass correlation coefficient of the differences between baseline and follow-up for each dimension.
SDxx: SD of the difference between the baseline and follow-up groups.
The MID is estimated as 1×SEM.18 The MID can be used to estimate the MCID. It is defined as the smallest difference in the score on an item that a patient can perceive as beneficial and that, in the absence of side effects or excessive cost, would lead to a change in patient management. The MID and MCID are usually similar and, in the absence of external criteria, MCID is considered to be equal to MID.
Floor or ceiling effects are considered if 15% of the patients obtain the minimum or maximum possible scores, respectively, for each dimension of the questionnaire.
Follow-upAll patients received programmed clinical follow-up 3, 6, and 12 months after ablation. Any visit to either a cardiologist or the emergency department was recorded in the patient's clinical history. Six months after the procedure, a 7-day Holter monitor was used to assess asymptomatic events. Oral anticoagulation was used for 3weeks after the electrophysiology study and thereafter according to the guidelines of the European Society of Cardiology.19
Statistical AnalysisAnalysis of the SF-36 scales was performed by t test and nonparametric Mann-Whitney test according to whether the data followed a normal distribution or not. The Kolmogorov-Smirnoff test was used to verify whether the data obeyed a normal distribution and the Levene test to assess the homogeneity of the variances. The Wilcoxon test for paired samples was used to compare the results obtained on the scales at baseline and during follow-up after ablation. Survival free of arrhythmias (AFib or AFl) was analyzed using Kaplan-Meier curves.
Ethical ConsiderationsThe study was performed in accordance with the principles of the Declaration of Helsinki (1975) and was approved by the Clinical Research Ethics Committee of Galicia. Signed informed consent was received from all patients.
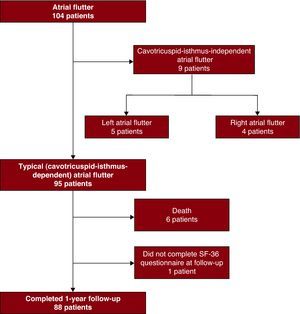
Results Clinical Characteristics of the PatientsOf the 104 patients consecutively referred to the cardiac electrophysiology laboratory for AFl, 95 had typical AFl (CTI dependent) and were included in the study (Figure 1). The baseline characteristics of the patients are shown in Table 2. Hypertension was reported in almost 50% of patients and 21% did not have known heart disease. The most frequent concomitant heart disease was hypertensive heart disease (39%) and 20% had signs of heart failure. One in every 4 patients was obese and 40% had significant respiratory disease. Significant arthritis was present in 24% of cases.
Figure 1. Flow diagram of the patients who underwent electrophysiology study for atrial flutter.
Table 2. Clinical Characteristics of the Patients.
| Age, years | 64±11 |
| Sex | |
| Men | 77 (81.1%) |
| Women | 18 (18.9%) |
| COPD | 20 (21.1%) |
| Hypertension | 47 (49.5%) |
| Smoking | 45 (47.4%) |
| Hyperlipidemia | 41 (43.2%) |
| Excessive alcohol consumption | 12 (12.6%) |
| Obesity | 25 (26.3%) |
| LVEF<50% | 22 (23.1%) |
| Hypertensive heart disease | 37 (38.9%) |
| Valve disease | 19 (20%) |
| Type of valve disease | |
| Moderate aortic stenosis | 4 (4.2%) |
| Mitral insufficiency | 12 (12.6%) |
| Grade II | 9 (9.5%) |
| Grade III | 3 (3.1%) |
| Tricuspid insufficiency | 3 (3.2%) |
| Grade III | 2 (2.1%) |
| Grade IV | 1 (1.1%) |
| Ischemic heart disease | 14 (14.7%) |
| Previous myocardial infarction | 10 (10.5%) |
| Heart failure | 18 (18.9%) |
| Dilated cardiomyopathy | 21 (22.1%) |
| Following heart surgery | 12 (12.6%) |
| Type of surgery | |
| Aortocoronary bypass | 8 (8.3%) |
| Pericardiectomy | 1 (1.1%) |
| Valvular | 3 (3.2%) |
| No heart disease | 20 (21.1%) |
| Cor pulmonale | 5 (5.3%) |
| Bronchial asthma | 5 (5.3%) |
| Definitive pacemaker carrier | 7 (7.4%) |
| Chronic kidney failure | 8 (8.4%) |
| Myotonic muscular dystrophy | 2 (2.1%) |
| Diabetes mellitus | 19 (20%) |
| Arthritis | 23 (24.2%) |
| Peripheral artery disease | 4 (4.2%) |
COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction.
Data are shown as n (%) or mean ±standard deviation.
Table 3 shows the characteristics of the arrhythmia. The most common type of AFl was paroxysmal (56%). Almost 40% of patients had previously received cardioversion (electrical or pharmacological). In 43% of cases, patients had concomitant AFib. Ablation was performed for a first episode of AFl in 44% of patients. Only 58% were receiving anticoagulation therapy prior to the procedure. In 15% of cases, AFl related to antiarrhythmic drugs was identified, mostly due to amiodarone. The incidence of tachycardia was 17%.
Table 3. Arrhythmia-Related Characteristics.
| Presenting form of flutter | |
| Paroxysmal | 53 (55.8%) |
| Persistent | 42 (44.2%) |
| Type of flutter | |
| Typical (anticlockwise) | 82 (86.3%) |
| Inverse typical (clockwise) | 6 (6.3%) |
| Both | 7 (7.4%) |
| Ventricular cycle, ms | 653±223 |
| Paroxysmal | 641±260 |
| Persistent | 686±192 |
| Duration of flutter, mo | 39.7±64.8 |
| No. of episodes of flutter | 3.7±3.7 |
| First episode of flutter | 42 (44.2%) |
| Previous electrical cardioversion | 22 (23.2%) |
| Atrial flutter | 15 (15.8%) |
| Atrial fibrillation | 3 (3.2%) |
| Both | 2 (2.1%) |
| Overstimulation | 2 (2.1%) |
| Previous pharmacological cardioversion | 15 (15.8%) |
| Previous anticoagulation | 55 (57.9%) |
| Subsequent anticoagulation | 63 (66.3%) |
| Previous atrial fibrillation | 41 (43.2%) |
| Sinus dysfunction | 15 (15.8%) |
| Long H-V interval | 18 (18.9%) |
| Ic flutter or amiodarone | 14 (14.7%) |
| Amiodarone | 11 (11.6%) |
| Flecainide | 3 (3.1%) |
| Tachycardia | 16 (16.8%) |
| Conduction disorder | 43 (45.3%) |
| First-degree atrioventricular block | 12 (12.5%) |
| Second-degree atrioventricular block | 2 (2.1%) |
| Bifascicular block | 4 (4.2%) |
| Trifascicular block | 1 (1.1%) |
| Right bundle branch block | 7 (7.4%) |
| Left bundle branch block | 1 (1.1%) |
| Left anterior hemiblock | 11 (11.6%) |
| Pacemaker during the procedure | 2 (2.1%) |
| Nonspecific | 3 (3.2%) |
| Antiarrhythmic | 54 (56.8%) |
| No. of antiarrhythmic drugs | |
| 0 | 41 (43.2%) |
| 1 | 40 (42.1%) |
| 2 | 11 (11.5%) |
| 3 | 3 (3.2%) |
Data are shown as n (%) or mean ±standard deviation.
The initial success rate for the procedure was 100%; bidirectional CTI conduction block was achieved in all patients. No in-hospital deaths occurred. Six patients died during follow-up, of 5 recorded causes: lung cancer, severe aortic stenosis, respiratory failure as a result of severe chronic obstructive pulmonary disease, respiratory infection, and 2 due to sudden death outside of hospital (1 of which was pulmonary thromboembolism). One patient could not complete the quality-of-life questionnaire during follow-up due to neurological sequelae following a cerebrovascular accident. Consequently, 88 patients satisfactorily completed the quality-of-life questionnaires at baseline and during follow-up.
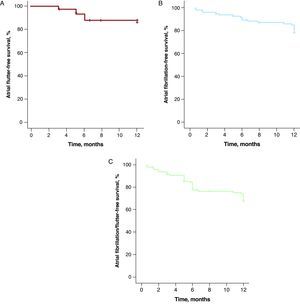
At 12-month follow-up, 13 cases of recurrence of typical AFl (14.6%) were recorded. In 12 cases, reablation of the CTI was carried out and in 1 case electrical cardioversion was used; 24 patients (25%) had episodes of AFib during follow-up, compared with 41 (43%) who had a history of AFib prior to the electrophysiology study. Of the 24 patients who had episodes of AFib during follow-up, 20 (83%) had a prior history of AFib. At 1-year follow-up, AFl-free survival was 85% (Figure 2A), AFib-free survival was 80% (Figure 2B), and AFl/AFib-free survival was 67% (Figure 2C). Three patients had both types of arrhythmia. At 12-month follow-up, 81 patients (91%) were in sinus rhythm, 7 (8%) were in AFib, and 1 (1%) was in AFl.
Figure 2. Kaplan-Meier curve for survival free from arrhythmias over 12 months of follow-up in patients subjected to ablation of the cavotricuspid isthmus. A, Typical atrial flutter. B, Atrial fibrillation. C, Atrial fibrillation or flutter.
Sixty-six patients (66%) were receiving anticoagulation therapy at 1-year follow-up and 24 (27%) were receiving antiplatelet therapy. At baseline, 55 patients (58%) were receiving anticoagulation therapy.
At 1-year follow-up, 29 patients (31%) were receiving antiarrhythmic drugs, compared with 54 patients (57%) who received antiarrhythmia treatment prior to ablation.
Quality of LifeTable 4 shows the results on the SF-36 questionnaire at baseline and 1-year follow-up. At baseline, role-physical was the dimension that displayed the greatest reduction compared with the population mean (11.7 points). This was followed by role-emotional (9.7 points), vitality (8.3 points), and mental health (8.1 points). Bodily pain was the least-affected dimension. The PCS had a higher mean value than the MCS. The reduction in PCS and MCS was 5.7 points and 8.8 points, respectively.
Table 4. Quality of Life at Baseline and 1-Year Follow-up, Standardized and Normalized for the Spanish Population, Adjusted for Age and Sex.
| Baseline (n=95) | Follow-up (n=88) | |
| Physical functioning | 43.9 (11.7) | 54.5 (6.6) |
| Role-physical | 38.3 (11.4) | 49.9 (8.8) |
| Bodily pain | 47.8 (10.8) | 51.2 (9.6) |
| General health perceptions | 42.9 (9.4) | 49.5 (10) |
| Vitality | 41.7 (10.7) | 52.3 (9.7) |
| Social activity | 43.5 (15) | 50.6 (11) |
| Role-emotional | 40.3 (15.7) | 49.5 (12.1) |
| Mental health | 41.9 (11.3) | 50.1 (8.9) |
| PCS | 44.3 (10.1) | 52.2 (8.2) |
| MCS | 41.2 (14.5) | 49.4 (11.4) |
MCS, mental component summary; PCS, physical component summary.
Data are shown as mean (standard deviation).
At 1-year follow-up, the scores on all of the scales were close to or greater than 50. The mean PCS was higher than the mean MCS. A ceiling effect was observed in the physical function, role-physical, bodily pain, social activity, and role-emotional dimensions.
In a comparison between baseline and follow-up, large differences (>8 points) were observed in physical function, role-physical, vitality, role-emotional, mental health, and MCS, and moderate changes (>6 points) were observed for general health, social activity, and PCS.
The ES and SRM are shown in Table 5. There was a large effect (≥0.8) in the magnitude of the change following the procedure for the physical function, role-physical, general health, and vitality dimensions and for PCS. In the role-emotional, social activity, and mental health dimensions and the MCS, there was a moderate effect (≥0.5), and a small change was observed in the bodily pain dimension.
Table 5. Differences in Quality of Life Standardized and Normalized for the Spanish population; Effect Size, Standardized Response Mean, and Minimum Clinically Important Difference (n=88).
| Mean | ES | SRM | MCID (ES) | MCID (SRM) | |
| Physical functioning | 10.4 (10.1)* | 0.88 | 1.03 | 0.62 | 0.69 |
| Role-physical | 11.8 (12.1)* | 1 | 0.94 | 0.75 | 0.68 |
| Bodily pain | 3.4 (11.2) | 0.31 | 0.3 | 0.62 | 0.62 |
| General health perceptions | 6.9 (8.6)* | 0.74 | 0.8 | 0.56 | 0.54 |
| Vitality | 10.7 (10.6)* | 0.99 | 1.01 | 0.59 | 0.59 |
| Role-emotional | 9.9 (13.1)* | 0.63 | 0.76 | 0.64 | 0.7 |
| Social activity | 7.6 (11.6)* | 0.5 | 0.66 | 0.58 | 0.66 |
| Mental health | 8.5 (11.1)* | 0.74 | 0.77 | 0.65 | 0.66 |
| PCS | 7.8 (9.7)* | 0.78 | 0.8 | 0.62 | 0.58 |
| MCS | 8.9 (12.7)* | 0.6 | 0.7 | 0.55 | 0.64 |
ES, effect size; MCID, minimum clinically important difference; MCS, mental component summary; PCS, physical component summary; SRM, standardized response mean.
Quantitative variables are shown as means (standard deviation).
*P<.001.
The MCID was achieved on all dimensions except bodily pain and social activity, such that the change exceeded the minimum required to be clinically perceived by the patient on those dimensions. The PCS and MCS exceeded the threshold established for the MCID.
Patients with AFib significantly improved their quality of life (Table 6). No significant differences were observed between patients who developed AFib during follow-up and those who did not (Table 7). There were no significant differences in the improvement in quality of life between patients with a first episode of AFl and those with recurrent AFl.
Table 6. Differences in Quality of Life Between Follow-up and Baseline in Patients With Atrial Fibrillation Prior to the Procedure (A) and Patients With Atrial Fibrillation During Follow-up (B).
| A. Prior AFib (n=39) | B. AFib following ablation (n=23) | |||
| Difference | P | Difference | P | |
| Physical functioning | 23.1 (22.7) | <.001 | 24.1 (23.9) | <.001 |
| Role-physical | 38.2 (41.6) | <.001 | 34.3 (39.6) | .001 |
| Bodily pain | 7.1 (29.6) | .15 | 10.4 (31.6) | .22 |
| General health perceptions | 9.2 (20) | .004 | 10.2 (19.4) | .02 |
| Vitality | 23.1 (26.1) | <.001 | 20.4 (26.5) | .003 |
| Social activity | 17.4 (25) | <.001 | 16 (19.1) | .001 |
| Role-emotional | 35.9 (41.4) | <.001 | 34.8 (43.2) | .004 |
| Mental health | 18.8 (22.4) | <.001 | 13.2 (21.8) | .007 |
AFib, atrial fibrillation.
Data are shown as mean (standard deviation).
Table 7. Differences in Quality of Life Between Follow-up and Baseline According to Whether or Not Atrial Fibrillation Occurred During Follow-up.
| AFib (n=23) | No AFib (n=65) | P | |
| Physical functioning | 24.1 (23.9) | 24.5 (20.1) | .94 |
| Role-physical | 34.3 (39.6) | 48.8 (47.3) | .19 |
| Bodily pain | 10.4 (31.6) | 9.6 (32.2) | .91 |
| General health perceptions | 10.2 (19.4) | 17.1 (19.2) | .15 |
| Vitality | 20.4 (26.5) | 26.6 (23.1) | .32 |
| Social activity | 16 (19.1) | 16.7 (27.1) | .88 |
| Role-emotional | 34.8 (43.2) | 30.3 (39.8) | .66 |
| Mental health | 13.2 (21.8) | 19.4 (23.5) | .25 |
AFib, atrial fibrillation.
Data are shown as mean (standard deviation).
The Cronbach α internal consistency coefficient was 0.85 for the scales at both baseline and follow-up, indicating that the values were adequate for between-group comparisons.
DiscussionIn this study, we observed a clinically significant improvement in quality of life following CTI ablation in patients with typical AFl. This result was obtained despite the inclusion of patients with concomitant AFib (43%) and a single episode of AFl (44%) in the cohort. Statistically significant improvements were observed on all scales of the SF-36 questionnaire and on the PCS and MCS. When adjusted for the values obtained in the general population, no significant differences were observed in the bodily pain dimension. This is not surprising, since only 7 patients (7.4%) presented with chest pain as the predominant symptom, and substantial changes would therefore not be expected in this dimension.
In the analysis of the magnitude of the change, assessment of ESs standardized and normalized for the Spanish population adjusted for age and sex revealed a large effect on the PCS and a moderate effect on the MCS, indicating a more marked improvement in physical than mental health status. However, we consider the concept of MCID to be a more important expression of quality-of-life changes that facilitates clinical decision-making. The MCID is a parameter of HRQoL that allows determination of whether a perceived benefit in the health status of the patient is sufficient, in the absence of side effects or excessive costs, to justify a change in therapeutic management of the patient.16, 17 Thus, the magnitude of change in quality of life exceeded the threshold for the MCID on all dimensions except for bodily pain and social activity. Therefore, treatment by ablation of the CTI resulted in a change in health status that was perceived by the patient with a sufficient magnitude for it to be taken into consideration in all patients with typical AFl.
The use of MCID to express HRQoL results is novel in patients with cardiac arrhythmias and has direct clinical implications, since it does not assess whether radiofrequency catheter ablation of the CTI led to a statistically significant benefit in HRQoL but rather whether this benefit, in addition to being statistically significant, was sufficient to justify the consideration of CTI ablation as a therapeutic option for all patients with typical AFl.
In an earlier study of HRQoL in patients with typical AFl, Calkins et al.5 demonstrated benefits on 6 of 8 dimensions of the SF-36 questionnaire in a cohort of 150 patients with typical AFl who underwent CTI ablation with 6 months of follow-up. Improvements were not observed in the bodily pain and general health dimensions. In another cohort of 169 patients with typical AFl who also underwent catheter ablation with 6 months of follow-up, Feld et al.6 reported improvements on 7 of 10 scales on the SF-36 questionnaire. There were no improvements on the bodily pain, general health, and role-emotional dimensions, although the baseline values were already very high (73, 67, and 71 points on the transformed scale, respectively). The PCS and MCS adjusted to the American population was 42 and 50, respectively. In our cohort, the baseline values for bodily pain, general health, and role-emotional were lower (69, 44, and 59 points on the transformed scale, respectively). The PCS and MCS standardized for the Spanish population adjusted for age and sex was 44 and 41, respectively. The cohort reported by Feld et al.6 was thus less affected by arrhythmia fundamentally in the mental dimensions.
O’Callaghan et al.7 reported, from a series of 55 patients who underwent CTI ablation with 12 months of follow-up, not only an improvement in HRQoL and a reduction in the frequency and severity of symptoms but also a reduction in the number of hospital admissions and visits to the emergency department due to arrhythmia.
In another study, Lee et al.8 showed an improvement in general HRQoL using a different questionnaire in a series of 100 patients with 6 months of follow-up. In a multivariate analysis, they found that the presence of AFib prior to ablation was the only factor that was independently associated with a lower improvement in quality of life. In our cohort, patients with AFib significantly improved their quality of life during follow-up. Nevertheless, they had lower scores at the end of follow-up than patients who had not suffered AFib.
Studies of HRQoL following typical AFl that have used the SF-36 questionnaire differ in some ways from our study. First, for obvious reasons, we standardized and normalized the results for the Spanish rather than the American population. Second, we adjusted for age and sex. Age is the most important factor influencing HRQoL, such that older patients have a worse quality of life.14 On the SF-36 questionnaire, it has a particular influence on the physical dimensions, for which lower scores are obtained with increasing age. Men report better quality of life than women on the SF-36 questionnaire in general, and it is therefore essential to adjust for age and sex in order to accurately express the results.15 Finally, we have expressed the results in terms of MCID, which is a parameter that indicates the clinical benefit perceived by the patient following a therapeutic intervention.
LimitationsOne of the limitations of our study is the presence of a ceiling effect on the role-physical, bodily pain, social activity, and role-emotional dimensions at baseline and on the physical function, role-physical, bodily pain, social activity, and role-emotional dimensions during follow-up. In other words, it is possible that the differences obtained on these dimensions were underestimated and, therefore, that the benefit could be even greater. Furthermore, although not as important from the point of view of improvements in HRQoL, we should mention the presence of a floor effect at baseline on the role-physical and role-emotional dimensions.
Another limitation arises from the prospective nature of the study in a group of older patients with comorbidities. Thus, some patients were lost to follow-up due to death or inability of the patient to respond to a second questionnaire at 12 months. This limitation adds to another derived from the small number of patients recruited. The observational nature of the study, without randomization of the patients to treatment or placebo, precludes the establishment of a causal relationship. These results should therefore be confirmed in appropriately controlled studies.
Finally, we used a general questionnaire for the evaluation of quality of life and did not complement it with a specific questionnaire to assess quality of life in patients with cardiac arrhythmias.
ConclusionsAn improvement in quality of life was observed in a group of patients with typical AFl 1 year after radiofrequency catheter ablation of the CTI.
FundingThis study was partially funded by a grant from the Instituto de Salud Carlos III (redINSCOR [RD06/0003/0016 and RD06/0003/0008], redIAPP [RD06/0018/0006]). F. Gude received a grant (BAE09/90052) from Instituto de Salud Carlos III (Ministerio de Ciencia y Tecnología).
Conflict of interestNone declared.
Received 23 July 2010
Accepted 13 December 2010
Corresponding author: Unidad de Arritmias, Servicio de Cardiología, Hospital Clínico de Santiago de Compostela, Travesía de la Choupana s/n, 15706 Santiago de Compostela, A Coruña, Spain. Javier.Garcia.Seara@sergas.es