In patients with acute myocardial infarction, elevation of plasma glucose levels is associated with worse outcomes. The aim of this study was to evaluate the association between stress hyperglycemia and in-hospital mortality in patients with acute myocardial infarction with ST-segment elevation (STEMI).
MethodsWe analyzed 834 consecutive patients admitted for STEMI to the Coronary Care Unit of our center. Association between admission glucose and mortality was assessed with Cox regression analysis. Discriminative accuracy of the multivariate model was assessed by Harrell's C statistic.
ResultsEighty-nine (10.7%) patients died during hospitalization. Optimal threshold glycemia level of 140mg/dl on admission to predict mortality was obtained by ROC curves. Those who presented glucose ≥140mg/dl showed higher rates of malignant ventricular tachyarrhythmias (28% vs. 18%, P=.001), complicative bundle branch block (5% vs. 2%, P=.005), new atrioventricular block (9% vs. 5%, P=.05) and in-hospital mortality (15% vs. 5%, P<.001). Multivariate analysis showed that those with glycemia ≥140mg/dl exhibited a 2-fold increase of in-hospital mortality risk (95% CI: 1.2-3.5, P=.008) irrespective of diabetes mellitus status (P-value for interaction=0.487 and 0.653, respectively).
ConclusionsStress hyperglycemia on admission is a predictor of mortality and arrhythmias in patients with STEMI and could be used in the stratification of risk in these patients.
Keywords
Many studies have shown that an elevated plasma glucose level on admission is a major independent predictor of in-hospital and long-term outcome in patients with acute myocardial infarction (AMI), regardless of diabetes status.1,2,3,4,5 In a retrospective study, Stranders et al.6 observed that for each 18mg/dL of glucose increase, there was a 4% increase in mortality in non-diabetic subjects. The Cooperative Cardiovascular Project, the largest retrospective study in elderly patients hospitalized with AMI to date, demonstrated a significant 13%–77% relative increase in 30-day mortality and a 7%–46% relative increase in 1-year mortality, depending on the degree of hyperglycemia.7 In the randomized CREATE-ECLA trial, which evaluated patients with ST-segment elevation myocardial infarction (STEMI), the 30-day mortality rate was 6.6% among patients with baseline glucose in the lowest tertile, whereas those in the highest glucose tertile experienced a mortality rate of 14%.8
However, there are some gaps and discrepancies in the studies. These disappointments may be due to patient group heterogeneity, with different types of AMI STEMI vs. non-STEMI, different cut-off values for definition of stress hyperglycemia, or different treatment strategies, i.e., fibrinolytic therapy vs. percutaneous coronary intervention (PCI). High blood glucose levels on admission in STEMI affect the prognosis of patients without diabetes melllitus (DM); however, it is not an independent mortality risk factor in patients with DM treated with PCI.9 Moreover, there is currently no consensus about the precise glucose value that should be considered abnormal in these patients upon admission. Consequently, stress glucose cut-off varied from study to study and the proportion of patients with stress hyperglycemia ranged from 31% to 71% in non-diabetic patients and from 46% to 84% in diabetic patients.4 Apart from these considerations, the fact that the prognosis of patients without a prior diagnosis of DM but with increased blood glucose in AMI is similar or even worse than patients with DM is very interesting.7,9
On the other hand, the pathophysiologic mechanism underlying the association of hyperglycemia with mortality in patients with STEMI is not fully understood. When the sympathetic nervous system is stimulated during AMI onset, catecholamine-induced tissue lipolysis occurs with a release of free fatty acids, which may result in the myocardium no longer receiving the optimum balance of energy. The combination of catecholamine release, lipolysis and ischemic myocardium may lead to malignant arrhythmias and subsequent mortality.10
The aim of this study was to assess whether stress blood glucose level in patients with STEMI is of prognostic significance and could be used in short-term risk stratification in patients with known or unknown DM. Moreover, we sought to investigate the mortality impact of admission glycemia associated to malignant arrhythmias.
Materials and methods Patients SelectionWe prospectively analyzed a cohort of 834 consecutive patients admitted for STEMI to the coronary care unit (CCU) from January 2004 to December 2008. Patients with neoplasia (2 patients), active infections (20) or inflammatory diseases (2) were excluded. All patients included gave informed consent to participate in the study.
STEMI was defined based on the criteria established by current guidelines as persistent (≥30min) retrosternal pain associated to ST-segment elevation ≥0.1mV in two or more limb leads or ≥0.2mV in two or more pericardial leads, or acute bundle branch block (BBB).11,12
Demographic data were obtained from all patients. These included age, gender, body mass index and the prevalence of the cardiovascular risk factors DM, hypertension, smoking habits and dyslipemia. Additional clinical data included prior PCI or coronary by-pass graft surgery. The following complications were registered during hospitalization: sustained ventricular tachyarrhythmias requiring intervention (ventricular tachycardia or/and ventricular fibrillation), acute heart failure, recurrence of angina or myocardial infarction, and death. Heart failure was defined as progressive resting dyspnea associated with clinical signs of pulmonary congestion, based on Killip criteria.
All patients received recommended standard management for STEMI. Patients with contraindication for thrombolytic therapy were referred for urgent invasive angiography with the intention of performing primary PCI. Moreover, in the event of unsuccessful fibrinolytic therapy patients were treated with rescue PCI.
All diabetic and non-diabetic patients with hyperglycemia in the acute phase of STEMI were treated with subcutaneous short-acting insulin according to digital glycemia test. After discharge from the CCU, elevated glycemia was treated with long-acting insulin twice daily.
Definition of Diabetes and Hyperglycemia. Laboratory AnalysesDM was defined as prior history of diabetes obtained from hospital records, or when the patient reported receiving a diagnosis or pharmacologic treatment was started (oral hypoglycemic drugs or insulin). Because stress glucose cut-off values are poorly defined, our proposed glucose threshold on admission was based on optimizing the sum of sensitivity and specificity, derived from receiver operating characteristic (ROC) curves, which predict the development of the primary end point (in-hospital mortality).
All analyses were measured by conventional laboratory methods. Blood samples were collected from all patients in the emergency department. In addition, blood samples were collected within 24–48h of admission for total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol.
Continuous monitoring was performed in all patients during the CCU stay in order to determine any type of significant arrhythmia, malignant ventricular tachyarrhythmias (MVT) including ventricular tachycardia or ventricular fibrillation, paroxysmal or persistent atrial fibrillation, complicated intraventricular conduction defects (left or right BBB) and at least second degree atrioventricular block (AVB). During hospitalization, an electrocardiogram was performed every day and when the patient had symptoms.
Statistical AnalysisContinuous variables with abnormal distribution (Kolmogorov–Smirnov test) were transformed by neperian logarithm before analysis. Continuous variables were expressed as mean±standard deviation or standard error mean, determining the differences between groups by Student's t-test. The categorical variables were compared by chi-square analysis. The relationship between continuous variables was examined using the Pearson correlation co-efficient. ROC curve analysis was used to assess the ability of various levels of glucose on admission to predict mortality.
The significant variables in the univariate analysis were introduced in a multivariate logistical regression model to obtain the predictive variables of adverse outcomes. Indicators were entered into the full multivariate model if the P value of the univariate association was <.25. The final multivariate model was constructed by forward stepwise elimination of the least significant factors in the univariate analysis. The proportionality assumption for the hazard function over time was tested by Schoenfeld residuals. The model's discriminative ability was assessed by Harrell's C statistic and its calibration by the Gronnesby and Borgan test.13
Event rates for clinical outcomes were determined using the Kaplan–Meier method and compared using the log-rank test. A P-value of <.05 was considered statistically significant for all analyses. Data were analyzed using SPSS version 9.0 (SPSS Inc., Chicago, Illinois, USA).
Results Baseline CharacteristicsTable 1 describes the baseline characteristics of the population. The mean age of our sample was 64±13 years and 74% were males. Anterior STEMI was present in 505 (61%) patients and a history of DM as a cardiovascular risk factor in 274 (33%) patients. Median time from onset of chest pain to admission was 3h (range 1–48h), and patients were discharged from hospital after an average of 7 days (range 1–11 days). Among 280 patients who underwent in-hospital PCI, a primary PCI was performed in 79 (28%) patients and a rescue PCI after unsuccessful fibrinolytic therapy in 50 (18%) patients.
Table 1. Clinical Characteristics of Patients With ST-Segment Elevation Myocardial Infarction a
| Variable | Baseline characteristics |
| Number of patients | 834 |
| Age (years) | 64±13 (25–94) |
| Males | 619 (74%) |
| BMI (kg/m2) | 27±5 |
| NYHA>II | 21 (2.5%) |
| Anterior STEMI | 505 (61%) |
| Pain-emergency department (h) | 3 (1–48) |
| Killip class on admission >I | 222 (26%) |
| SBP (mmHg), admission | 130±31 |
| DBP (mmHg), admission | 78±18 |
| Heart Rate (bpm), admission | 79±22 |
| Cardiovascular risk factors | |
| Diabetes mellitus | 274 (33%) |
| Hypertension (≥140/90mmHg) | 443 (53%) |
| Smoking | 352 (42%) |
| Dyslipemia | 349 (41%) |
| Prior myocardial infarction | 183 (22%) |
| Familiar history | 72 (9%) |
| Prior PCI | 39 (5%) |
| Prior CABG | 19 (2%) |
| Glycemia, admission (mg/dL) | |
| Diabetes mellitus (274 patients) | 226±108 |
| Non-diabetic (560 patients) | 149±57 |
| Glycemia on admission ≥140mg/dL | 455 (54%) |
| Maximum serum biomarkers | |
| White blood cell counts (109/L) | 11 390±4.111 |
| TnI (ng/mL) | 59±54 |
| CK MB (ng/mL) | 195±201 |
| CRP (mg/L) | 52±60 |
| Creatinine, admission (mg/dL) | 1.16±0.9 |
| GFR (MDRD), admission | 64±28 |
| Prior pharmacological medication | |
| AAS | 22 (9%) |
| ACEI | 356 (43%) |
| Statin | 337 (40% |
| Antidiabetic drugs | 210 (25%) |
| Insulin | 66 (8%) |
| Concomitant hospital medication | |
| Low molecular weight heparin | 508 (61%) |
| AAS | 802 (96%) |
| Clopidogrel | 340 (43%) |
| ACEI | 460 (55%) |
| Beta blockers | 393 (47%) |
| Diuretics | 258 (31%) |
| Inotropics and vasopressor agents b | 50 (6%) |
| Statins | 482(58%) |
| Insulin | 310 (37%) |
| Reperfusion therapy | |
| Fibrinolysis agents | 435 (52%) |
| Primary PCI | 79 (9.5%) |
| Rescue angioplasty | 50 (6%) |
| LVEF (447 patients) | 51±14 |
| All-cause in-hospital mortality | 89 (10.7%) |
| Hospital stay, days (median) | 7 (1–18) |
ACEI, angiotensin-converting-enzyme inhibitor; ASA, acetylsalicylic acid; BMI, body mass index; CABG, coronary artery bypass graft; CK MB, creatine kinase MB; CRP, C-reactive protein; DBP, diastolic blood pressure; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; MDRD, Modification of Diet in Renal Disease; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST elevation myocardial infarction; Tn I: troponin I.
a Data express n (%), mean ± standard deviation and median (interval).
b Inotropics and vasopressor agents: dopamine, dobutamine, levosimendan.
Patients with known DM more often had a history of hypertension (39% vs. 26%, P=.001) and dyslipemia (46.7% vs. 39.5%, P=.046). Value of glycemia on admission was higher in patients with DM (226±108mg vs. 149±57mg, P=.001). Glycemia ≥140mg/dL was present in 79.8% of diabetic and 42.8% of non-diabetic patients (P<.001). In-hospital mortality was similar in patients with or without DM (11.7% vs. 10.2%, P=.510).
In-Hospital EventsMVT were present in 197 patients (24%), ≥2nd degree AVB in 59 patients (7%), new BBB in 30 patients (4%) and atrial fibrillation in 61 patients (7%). Eighty-nine patients died before hospital discharge (10.7%).
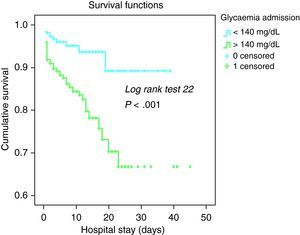
The best cut-off of glycemia on admission was 140mg/dL, with 80% sensitivity, while specificity was no higher than 50% (area under curve =0.697; P<.001). Mortality in patients with glycemia on admission ≥140mg/dL was 15% vs. 5% in patients with glycemia <140mg/dL (P<.001). Figure 1 shows the Kaplan–Meier curve according to the 140mg/dL glycemia cut-off in all patients with STEMI. Difference between the curves was statistically significant with a log rank test=22.3, P<.001.
Figure 1. Cumulative survival in patients with ST-segment elevation myocardial infarction by glycemia on admission.
Unadjusted relative risk for in-hospital mortality is shown in Table 2. It is important to observe that neither reperfusion therapy by primary PCI nor thrombolytic therapy were associated with a decrease in mortality.
Table 2. Unadjusted Relative Risk of In-Hospital Mortality in Patients With ST Elevation Myocardial Infarction.
| Variables (admission) | OR (95% CI) | P-value |
| Anterior STEMI | 1.45 (1.03–2) | .021 |
| Gender female | 1.9 (1.46–2.5) | .0001 |
| Age ≥70 years | 2 (1.76–2.4) | .0001 |
| Diabetes mellitus | 1 (0.8–1.2) | .5 |
| Hypertension | 1.28 (1.09–1.5) | .008 |
| Smoker | 0.39 (0.25–0.62) | .001 |
| Killip >I | 3 (2.5–3.8) | .001 |
| SBP ≥60mmHg | 3.6 (2.5–5) | .001 |
| Thrombolytic therapy | 1 (0.8–1.2) | .9 |
| Primary PCI | 0.56 (0.23–1.3) | .1 |
| Creatinine ≥1.4mg/dL | 3.6 (2.6–5) | .001 |
| LVEF ≤40% (124/447 patients) | 3.5 (2.8–4) | .001 |
| Glycemia≥140mg/dL | 1.5 (1.34–1.7) | .001 |
| White blood cell count >10,000c/mL | 1.7 (1.1–2.4) | .001 |
CI, confidence interval; LVEF, left ventricular ejection fraction; OR, odds ratio; PCI, percutaneous coronary interventions; SBP, systolic blood pressure; STEMI, ST elevation myocardial infarction.
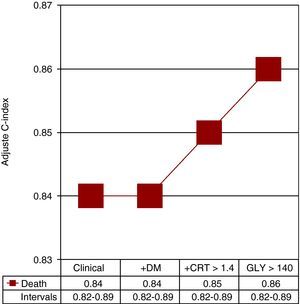
Multivariate analysis is shown in Table 3. The baseline covariates included in the final model were Killip >I, age ≥70 years and serum creatinine ≥1.4mg/dL. Interactions between glycemia, both continuous and dichotomized, and DM were not significant (P=.487 and .653, respectively), indicating no differential prognostic effect attributable to glycemia according to status. Figure 2 shows the estimated contribution of each clinical factor and independent biomarker in the model for predicting mortality after adjustment for potential confounders’ parameters.
Table 3. Multivariate Logistic Regression Predictors of Mortality in Patients With ST Elevation Myocardial Infarction.
| Variable | HR (95% CI) | P-value |
| Killip >I | 3.5 (2.18–5) | .0001 |
| Age ≥70 years | 1.8 (1.1–3.2) | .02 |
| Creatinine ≥1.4mg/dL | 2.2 (1.4–3.4) | .005 |
| Glycemia ≥140mg/dL | 2 (1.2–3.5) | .008 |
CI, confidence interval; HR, hazard ratio.
Figure 2. Logistic regression model in the stratification of all patients with ST-segment elevation myocardial Infarction. Adjusted Harrel's C statistic was calculated for death, introducing significant unadjusted clinical and analytical parameters on admission. Note that when diabetes mellitus (DM) was added to the model, adjusted Harrel's C statistic was not modified. CRT, creatinine; GLY, glycemia.
Harrell's C statistic of the multivariate models that included glycemia either as a binary or continuous variable showed a high discriminative ability (0.825 and 0.828). The Gronnesby and Borgan test of goodness-of-fit showed a good calibration of the model (P=.460).
The incidence of arrhythmias was more frequent in patients with glycemia ≥140mg/dL than in those with glycemia <140mg/dL: MVT was present in 28% vs. 18% (P=.001), new AVB in 9% vs. 5% (P=.05), and complicative BBB in 5% vs. 2% (P=.005), respectively. Atrial fibrillation was more prevalent in patients with hyperglycemia, but did not reach statistical significance (8% vs. 6%) (P=.2).
As expected, patients with ventricular tachyarrhythmias, new AVB, atrial fibrillation and complicative BBB exhibited higher mortality than patients without rhythm disturbances. Thus, these arrhythmias were associated with mortality regardless of type of hospitalization. In total, 89 patients died before hospital discharge (10.7%) and previous MVT was present in 48 (54%) patients, vs. 149 of the 743 surviving patients (20%), AVB in 17 (19%) vs. 42 (6%), atrial fibrillation in 21 (23%) vs. 40 (5%), and a new BBB in 14 (16%) vs. 16 (2%) patients, respectively, (P=.001 in all cases). In addition, patients with MVT and atrial fibrillation had higher maximum troponin I levels (77±45ng/mL vs. 57±54ng/mL; P=.001 and 76±46ng/mL vs. 58±54ng/mL; P=.012 respectively), during the hospital stay.
DiscussionThe present study, based on a non-selected cohort of patients with STEMI, highlights the relevance of stress hyperglycemia on admission to accurately identify a group of patients at high risk for short-term outcomes. This relationship was unaffected by diabetic status.
The multinational Euro Heart Survey pointed out that normal glucose regulation is less common than abnormal glucose regulation in patients with unstable coronary artery disease.14 The proportion of patients with stress hyperglycemia ranged from 31% to 71% in non-diabetic patients and from 46% to 84% in diabetic patients, similar to our observations.15 Hyperglycemia is present in up to 50% of all STEMI patients, whereas a previous diagnosis of DM is found in only 20%–25% of STEMI patients. When oral glucose tolerance testing is performed, the prevalence of type 2 DM or impaired glucose tolerance may be as high as 65% in patients with myocardial infarction without prior DM.16
Unlike fasting glycemia, which is well defined, stress glucose concentration cut-off values at the first hours after admission has not been completely defined because there is no unanimity of opinion.17 In a systematic overview of 11 studies reporting outcomes, Capes et al.4 observed that the threshold glucose concentration used to define stress hyperglycemia ranged from 6.7mmol/L to 11.0mmol/L (118mg/dL–198mg/dL) or from 6.1mmol/L to 8.0mmol/L (110mg/dL–145mg/dL) the morning after admission. Pertursson et al.18 stratified patients into quartile groups defined by admission plasma glucose: hyperglycemia was defined as plasma glucose >9.4mmol/L, which was the cut-off value for the fourth quartile in patients with acute coronary syndrome. Timmer et al.19 categorized patients according to glucose level at admission based on the values reported by the American Diabetes Association for diagnosing impaired glucose tolerance.20 In a large cohort study with patients presenting acute coronary syndrome, Foo et al.21 observed a higher risk with admission glucose concentration >10mmol/L (180mg/dL). In our experience, the optimal cut-off to predict in-hospital mortality, optimizing the sum of sensitivity and specificity, was a glucose of 140mg/dL.
The relationship between admission hyperglycemia and short-term mortality in subjects without known DM after AMI has been well documented.3,6,13,21,22 Increased glucose levels on admission (ranging from 110 to 144mg/dL) (6.1–8.0mmol/L) conferred an almost 4-fold risk of death in patients without known DM after an AMI.4 An increase of 18mg/dL (1mmol/L) in glucose levels was associated with a 4% increase of mortality risk in non-diabetic patients in a retrospective study.6 In the present study, our findings highlight a 2-fold increase in mortality risk with hyperglycemia after STEMI, as an additive to clinical parameters (Figure 2). Moreover, it is of interest to note that the probability of mortality was not modified when DM was added in our model, indicating that the predictive influence of DM was marginal. Similar results were found by Pinto et al.23 in a subgroup of patients in CLARITY-TIMI 28 study trial. In 1027 patients with STEMI treated with PCI, with 26% incidence of DM, Gasior et al.9 observed that an 18mg/dL increase of the baseline glucose level on admission was an independent prognostic factor of mortality.
It is difficult to explain why DM is only a mere testimonial role in the outcomes of these patients. The definition of stress hyperglycemia is intrinsically difficult in patients with DM because these patients are more likely to receive insulin or oral antidiabetic drugs for hyperglycemia before experiencing an AMI. This treatment may reduce the release in free fatty acids during an AMI, promote myocardial uptake of glucose for anaerobic metabolism, and decrease coagulability because of reduced production of thromboxane A.24 Correct treatment of these patients would explain fewer cardiovascular events and a better prognosis compared to undiagnosed DM patients with hyperglycemia and without treatment.25,26
The pathophysiologic mechanism underlying the association between hyperglycemia and mortality in patients with STEMI is not fully understood. Twenty-five years ago, Oswald et al.22 observed that the concentration of cortisol, epinephrine and norepinephrine were the main determinants of plasma glucose levels measured in non-diabetic patients with AMI. Recently, Kadry et al.27 observed a strong interaction between blood glucose concentration and presence of left ventricular failure on admission. However, the fact that hyperglycemia was a prognostic indicator in our patients, additive to initial heart failure, suggests the possibility that it was an important outcome factor, rather than a simple consequence of a larger or smaller infarct size. Several mechanisms would have been involved. There is strong experimental and clinical evidence that hyperglycemia per se may be detrimental. Acute hyperglycemia attenuates endothelium-dependent vasodilatation in humans in vivo, abolishes the effect of ischemic preconditioning, and induces oxidative stress affecting platelet function coagulation and fibrinolysis.23,28,29 Although this increase in mortality is likely due in part to resistance to fibrinolysis, an increase in mortality in hyperglycemic patients undergoing primary PCI, where vessel patency is nearly universal, has been reported.9,23,30 Timmer et al.31 observed that in the acute stage of myocardial infarction, hyperglycemia is a predictor of impaired coronary flow before reperfusion therapy. Iwakura et al.32 showed a strong association between admission serum glucose concentrations and the occurrence of a non-reflow phenomenon after angioplasty. Fefer et al.33 explored the possible reasons for this association by integrating clinical and angiographic data in relatively homogenous patients with STEMI undergoing primary PCI and observed that increased fasting glucose and previously diagnosed DM were associated with less spontaneous reperfusion and after primary PCI, resulting in worse clinical outcomes.
An interesting fact observed in our study is the association between MVT and hyperglycemia on admission. The mechanisms leading to ventricular fibrillation during acute myocardial ischemia are ill understood. An immediate response to the initial symptoms of an acute coronary syndrome is a rapid and marked increase in catecholamine release, which leads to adipose tissue lipolysis with an acute increase in plasma concentrations of free fatty acids, suppression of insulin activity and a reduction in myocardial glucose uptake. The utilization of free fatty acids instead of glucose by the ischemic myocardium could precipitate regional oxygen or energy crises, and may lead to damaged cardiac-cell membranes, calcium overload and arrhythmias preceding mortality in many patients.34 There is already experimental and clinical evidence to support that antilipolytic drugs decrease the incidence of ventricular fibrillation, although their potential has not been explored extensively.35
Finally, many of the non-diabetic patients who develop stress hyperglycemia are likely to be dysglycemic when not stressed. Norhammar et al.15 found that 65% of non-diabetic patients with glycemia >11mmol/L had undiagnosed DM or impaired glucose tolerance. A similar finding was recently observed by Meisinger et al.36 during a mean follow-up of 4.7 years. However, a diagnosis of DM cannot be confirmed in >50% of patients who initially present with severe increase of blood glucose (>200mg/dL) at time of AMI, further strengthening the notion that STEMI induces systemic changes that lead to transient impairment of glucose tolerance.37 Fewer than 20% of patients with STEMI enrolled in the CLARITY-TIMI 28 trial had a previous diagnosis of diabetes, but >65% of patients presented abnormal glucose levels that were associated with adverse outcomes.23
LimitationsFirst, due to the observational nature of this study, the possibility of selection bias and/or residual confounding from unknown or unmeasured covariates cannot be excluded. Second, this is a single-center observational study, so we should be cautious in hypothesizing about the mechanisms involved and the generalizability of our conclusions to other populations.
ConclusionsHyperglycemia on admission is a strong predictor of mortality in patients with STEMI and could be used in the risk stratification of these patients. Moreover, an association between high serum levels of glucose on admission and arrhythmias during hospitalization has been observed.
Conflicts of interestNone declared.
Acknowledgements
We wish to thank Miss Chris Guevara (Mercé V. Electromedicina S.L.) who was involved in translation support.
Received 18 May 2010
Accepted 25 August 2010
Corresponding author: Unidad Coronaria, Hospital Clínico Universitario, Avda. Blasco Ibáñez 17. 46010 Valencia, Spain. sanjuan_raf@gva.es