The growth in the use of implantable cardiac devices has inevitably resulted in an increasing number of individuals at risk of device-related infection. Registry data from the Spanish Society of Cardiology show progressive growth in the number of primary implants over the past 10 years.1 In addition to their cumulative risk of events, device carriers are at risk of infection associated with device-related procedures, such as generator or lead replacement or pacemaker/defibrillator upgrades to cardiac resynchronization therapy. Furthermore, patients facing these risks continue to grow older and accumulate comorbidities.
Intracardiac device infection can arise through a number of mechanisms. The most common is colonization of the prosthetic material by direct inoculation at the time of implantation or during subsequent manipulations. The second most common infection route is colonization by blood-borne bacteria transported from a distant infection site, such as a vascular catheter. For less common mechanisms such as contact infection, the evidence is anecdotal.
The risk factors for intracardiac device infection are known. Some are related to baseline patient characteristics, such as obesity, diabetes mellitus, and kidney failure. Others are related to device characteristics, such as generator size and the number of leads. Another set of risks is related to surgical procedures, including hemostatic control, the number of previous surgical interventions, and abandonment of unused leads. Some of these risk factors are modifiable, but unfortunately most are not. In this setting, a thorough risk assessment of each patient can play an invaluable role in preprocedure decision-making (for example, on the ability of antibiotic and antiseptic treatment to reduce the risk of wound colonization in patients with a methicillin-resistant Staphylococcus aureus infection). Similarly, individual risk assessment can inform decision-making about closer clinical management after the intervention (for example, patients with major postimplantation bleeding).
In a recent article published in Revista Española de Cardiología, Calderón-Parra et al. propose a score scale to identify patients at high risk of device infection and who are potential candidates for intensive preventive measures.2 Although the study goal was clearly valid, in my opinion, 2 features of the study make it hard to draw firm conclusions from the results.
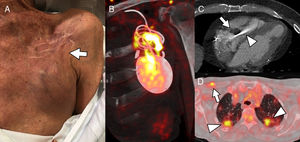
The first issue is the definition of infection. Intracardiac device infection has a very broad clinical spectrum due to the diversity of reported infection mechanisms and the commonly involved microorganisms (usually gram-positive cocci) (Figure 1).3 Sometimes the only sign of low-grade infection by coagulase-negative Staphylococci or Propionibacterium acnes is retraction of the skin surrounding the generator. At the other extreme is infective endocarditis resulting from S. aureus infection of the device leads and heart valves, leading to pulmonary embolism and respiratory failure. Despite the authors’ claims, there are no specifically modified Duke criteria for the diagnosis of infective endocarditis associated with intracardiac devices.4 The lack of a standard definition of local infection of the extravascular device components makes it highly likely that intravascular infections are overrepresented in the study by Calderón-Parra et al.; moreover, this likelihood is increased by the retrospective study design and the extensive experience of the study center in the treatment of infective cardioditis.5 A high proportion of intravascular infection in the study population could explain the relatively high mortality, which is comparable to that detected in a previous study of device-related endocarditis.6
A, Patient 1. The patient was fitted with an implantable cardioverter-defibrillator (ICD) 7 months before reporting pain in the generator pocket, with no fever or other symptoms; blood cultures were negative. The image shows mild skin retraction in the region above the pocket (arrow), with no obvious signs of inflammation. B, Coronal oblique plane reconstruction of cardiac positron emission tomography/computed tomography (PET/CT) with 18-fluorodeoxyglucose (18F-FDG), showing heterogeneous tracer capture around the generator and the lead regions close to the pocket, indicating infection of the ICD; device cultures were positive for Staphylococcus caprae. C, Patient 2. Cardiac CT shows extensive vegetation (arrow) in the intraventricular segment of an abandoned pacemaker lead (arrowhead). D, Patient 3. 18F-FDG capture on PET/CT reveals pacemaker infection in the device leads (arrow) and the presence of bilateral septic pulmonary emboli (arrowheads).
The aim of the risk score proposed by Calderón-Parra et al.2 was to identify patients who would benefit from special preventive measures, such as the placement of an antibiotic-impregnated envelope at the time of device implantation. This being the case, the study should have been restricted to assessment of the infection risk associated with the implantation procedure, and should thus have excluded patients with intravascular infections primarily originating in another location. Nevertheless, this issue was not considered in the study design. The high rate of endocarditis in the study population thus raises reasonable doubts about the suitability of the selected study objective.
The second study feature that needs to be considered is the assumption that implanting a device enclosed in an antibiotic-impregnated envelope is cost-effective in patients with a high risk of infection. Local antibiotic administration is an attractive and established prophylactic strategy; however, there is no solid evidence supporting its use in the current setting. The article by Calderón-Parra et al.2 was reviewed for publication in Revista Española de Cardiología before publication of the WRAP-IT study findings.7 This multicenter randomized controlled trial showed that an antibiotic-eluting envelope significantly reduced the rate of device infection in the first year after implantation (25/3495 among those receiving a envelope vs 42/3488 in the control group; hazard ratio [HR]=0.60; 95% confidence interval [95%CI], 0.36-0.98; P=.04). However, these data should be interpreted with caution. First, patients were included in the WRAP-IT study according to the type of procedure, without considering their baseline characteristics; therefore, the strategy does not address the same concerns as that by Calderón-Parra.,2 which are more in line with daily clinical practice. Moreover, the trial results indicate that avoiding a single infection episode would require the implantation of 205 envelopes; given the unit cost (€1150), this cannot become a routine strategy. Second, although the intervention and control groups were generally well balanced for comorbidities, immunosuppression therapy was more frequent in the control group (2.4% vs 1.4%; P<.05). Immunosuppression is a known risk factor for infection, and this between-group difference could thus indicate that patients receiving an antibiotic envelope already had a lower risk of infection. Finally, although the total number of infections was lower in the intervention group, this effect was exclusively due to lower generator-pocket infection; the number of infections due to bacteremia or endocarditis was surprisingly higher in the intervention group, a particularly worrying result with no apparent explanation. What these results tell us is that we need a more detailed analysis to clarify which patients, if any, could benefit from this procedure. Whatever the eventual outcome of such an analysis, it cannot be stressed enough that the introduction of a new preventive strategy should never replace or weaken other standard measures.
The prophylactic antibiotic therapy used at the study center was 400mg teicoplanin plus 80mg gentamicin via a single intravenous dose during the hour before surgical incision. This was supplemented with local irrigation of the wound with 2g cefazolin. More than 75% of intracardiac device infections are monomicrobial and caused by gram-positive coccal bacteria; gram-negative bacilli account for fewer than 10% of infections.7 Thus, although teicoplanin plus gentamicin is a recommended treatment in clinical practice guidelines,3 in the absence of multidrug resistance or high-level infection with gram-negative bacilli, prophylaxis could be achieved with a single dose of a cephalosporin (usually 2g cefazolin). A second antibiotic dose is justified in patients undergoing lengthy procedures or with substantial blood loss, as long as prophylaxis is not prolonged beyond 24hours. The available scientific evidence does not support local antibiotic irrigation.
The treatment of intracardiac device carriers is complex and requires highly specialized personnel. Considering only the technical issues, it takes years of experience to acquire the required skill levels in aseptic technique and hemostatic control, while the removal of infected devices requires the use of sheaths and stylets that are not available in all health care settings. Treatment options are influenced by a diverse array of factors, including the infection site (intravascular vs extravascular and occurrence with or without endocarditis), the indication for the device and the degree of dependency on it, and the identity of the causative infective agent. For these reasons, it is advisable to centralize procedures at referral hospitals with multidisciplinary teams experienced in the treatment of intravascular infections.
The baseline characteristics of the patients attending our clinics are unlikely to improve in the coming years, and health care resources are limited. The combined cost of implanting a device and treating a subsequent device infection should prompt us to consider which patients truly benefit from these treatments. This analysis can be assisted by cost evaluation models as long as these are used not only for regulatory purposes, but also as a tool for translational research, defined as the application of knowledge generated in clinical trials or epidemiological studies to daily clinical practice and decision making. An example is the updating of the 2011 analysis by the Canary Islands Health Service Assessment and Planning Service.8 This ongoing project will allow sensitivity analysis of the value of incorporating preventive strategies such as those proposed in the WRAP-IT study7; moreover, this analysis will help to define the number and risk profile of patients who require treatment so that the potential benefit and cost of the preventive strategy offset the injury and cost associated with device infection.8
CONFLICTS OF INTERESTNone declared.
Figure 1 was prepared by Dr Albert Roque, a member of the Cardiovascular Imaging Unit in the Radiology Department at Vall d’Hebron University Hospital.