Transthyretin cardiac amyloidosis (ATTR-CA) is a frequent cause of heart failure with preserved ejection fraction (HFpEF). This study sought to determine the prevalence of ATTR-CA among HFpEF patients in a multicenter nationwide study.
MethodsConsecutive ambulatory or hospitalized patients aged ≥ 50 years with HFpEF and left ventricle hypertrophy ≥ 12mm were studied at 20 Spanish hospitals. Screening for cardiac amyloidosis was initiated according to the usual clinical practice of each center. Positive scintigraphs were centrally analyzed.
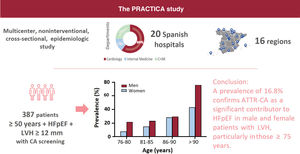
Results422 patients were included, of whom 387 underwent further screening for cardiac amyloidosis. A total of 65 patients (16.8%) were diagnosed with ATTR-CA, none below 75 years. There was an increase of prevalence with age. Of them, 60% were male, with a mean age of 85.3±5.2 years, mean left ventricle ejection fraction of 60.3±7.6% and a mean maximum left ventricle wall thickness of 17.2 [12-25] mm. Most of the patients were New York Heart Association class II (48.4%) or III (46.8%). Besides being older than non-ATTR-CA patients, ATTR-CA patients had higher median NT-proBNP levels (3801 [2266-7132] vs 2391 [1141-4796] pg/mL; P=.003). There was no statistical difference in the prevalence of ATTR-CA by sex (19.7% for men and 13.8% for women, P=.085). A ∼7% (4/56) of the patients exhibited a genetic variant (ATTRv).
ConclusionsThis multicenter nationwide study found a prevalence of 16.8%, confirming that ATTR-CA is a significant contributor to HFpEF in male and female patients with left ventricle hypertrophy and more than 75 years.
Keywords
Cardiac amyloidosis (CA) is a fatal disease caused by the deposition of amyloid fibers in the heart, resulting in infiltrative restrictive cardiomyopathy.1 More than 98% of cases of cardiac amyloidosis are caused by the deposition of fibrils of immunoglobulin light-chains (AL) amyloidosis or transthyretin (ATTR) amyloidosis.2 The signs and symptoms of these etiologies are similar but survival differs: median survival in ATTR-CA is approximately 4 years, but can be less than 6 months in untreated AL.3
One of the main problems of CA is its late diagnosis, which is directly related to patient survival.4 This is largely because this disease mimics other more common forms of left ventricular hypertrophy (LVH), such as hypertensive or hypertrophic cardiomyopathy.5 Correctly diagnosing these patients is indispensable since their survival is less favorable than that with other cardiomyopathies.6 ATTR-CA is known to place a high burden on health care systems. Patients with this disease have more day care visits and a higher number of heart failure (HF) hospitalizations than patients with HF without ATTR-CA.7 However, correct diagnosis of ATTR-CA increases survival and reduces cardiovascular hospitalizations, as well as resulting in savings for the national health system compared with nondiagnosis.8
A publication by several Italian referral centers has shown that half of the wild-type ATTR-CA (ATTRwt-CA) diagnoses occurred in a HF setting.9 One of the most common clinical phenotypes of ATTR-CA is HF with preserved ejection fraction (HFpEF).1,9 Diagnosis of this entity has increased over time.10 In 2021, the European Society of Cardiology (ESC) Guidelines highlighted the importance of identifying the underlying causes of HFpEF and recommended considering CA in its differential diagnosis.11
Several single-center studies have reported that the prevalence of ATTR-CA is between 5% and 20% in patients with HFpEF, applying several ages, definition of ejection fraction, and left ventricular (LV) wall thickness thresholds.12–17 The first multicenter prospective study of the prevalence of ATTR-CA in older patients with HF and any ejection fraction was published in 2023.18 The are no multicenter studies evaluating the prevalence of ATTR-CA among HFpEF patients, as defined by ejection fraction (≥ 50%) and the currently recommended LV wall thickness threshold for CA suspicion (≥ 12mm).
Therefore, we aimed to evaluate the prevalence of ATTR-CA in patients with HFpEF (≥ 50%) and LVH ≥ 12mm through a nationwide prospective study in Spain carried out mainly in cardiology departments.
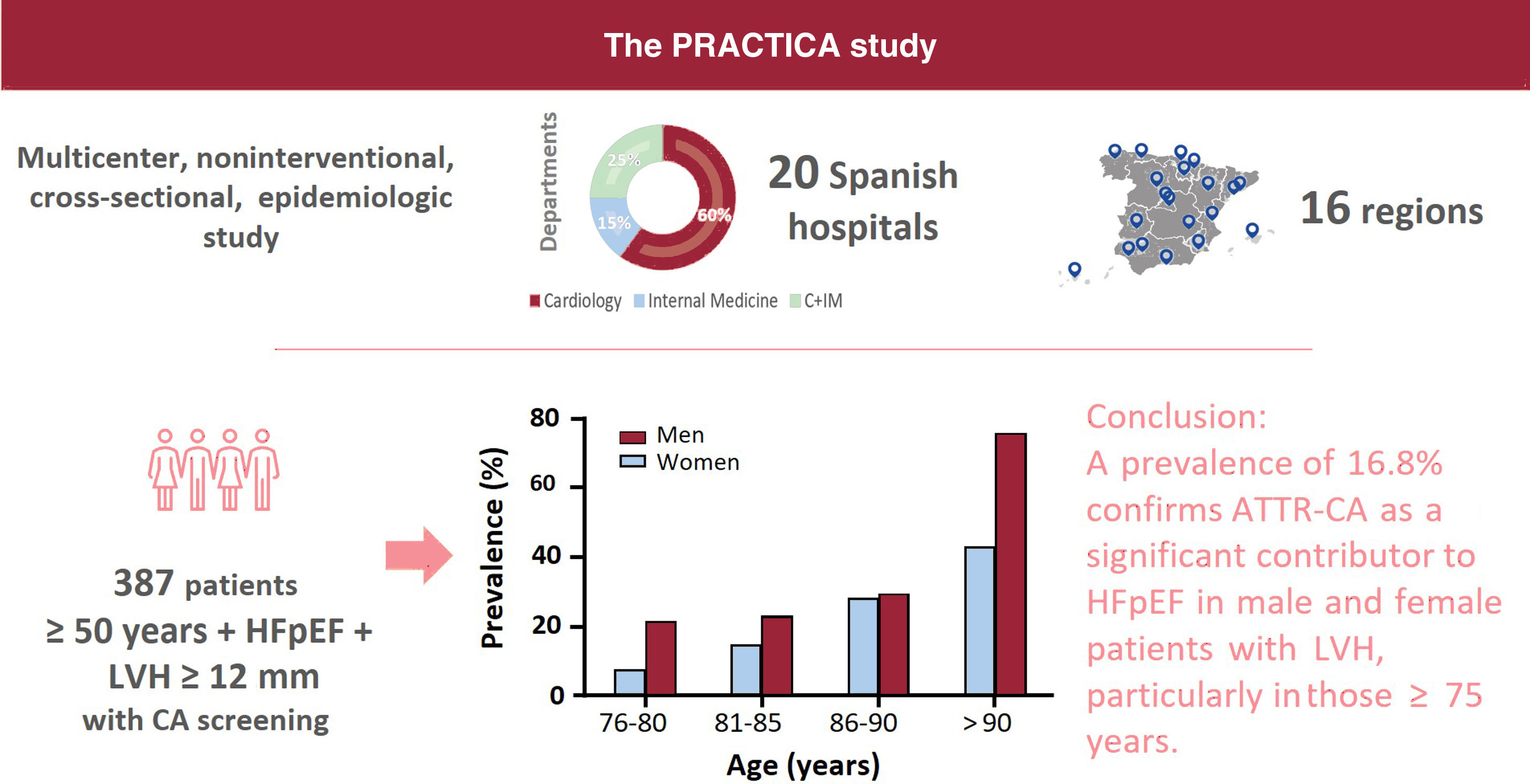
METHODSThe PRACTICA study is a multicenter, noninterventional, cross-sectional, epidemiologic study conducted at the HF departments of 20 Spanish hospitals distributed throughout 16 Spanish regions to ensure a good nationwide representation. Among these 20 hospitals, 17 cardiology and 8 internal medicine departments were involved (both departments participated in 5 centers). The principles of the Declaration of Helsinki were followed. The study protocol, amendments, and informed consent were reviewed and approved by independent ethics committees from each participating center. All patients provided written informed consent before study enrollment.
Study populationThis study included ambulatory or hospitalized patients aged ≥ 50 years with HFpEF and LVH ≥ 12mm, who were consecutively enrolled between December 2018 and May 2021. The participating centers and principal investigators are listed in table 1 of the supplementary data.
Inclusion criteria included the following: men and women ≥ 50 years; diagnosis of HFpEF according to ESC criteria at the time of study initiation,19 defined as left ventricular ejection fraction (LVEF) ≥ 50%, high levels of natriuretic peptides (BNP> 35 pg/mL and/or NT-proBNP> 125 pg/mL), and relevant structural heart disease (LVH and/or left atrial enlargement) and/or diastolic dysfunction; at least 1 previous admission for HF in the last 24 months, and evidence of LVH ≥ 12mm on echocardiography.
Exclusion criteria included the presence or history of significant coronary artery disease in at least 1 main coronary artery; the presence or history of significant valve disease; patients with a confirmed diagnosis of the origin of hypertrophic cardiomyopathy, or a restrictive cardiomyopathy other than CA (cardiomyopathy with a confirmed gene variant, myeloma, Fabry disease, sarcoidosis, any type of amyloidosis, etc); and withdrawal of informed consent.
ProtocolPatient information was obtained from medical records and patient interviews at the recruitment visit. Screening was performed according to clinical practice at each center (regarding the number/type of tests and order of request) at the time of the study. No data were collected on why certain tests were or were not performed. Screening could include imaging with 99mTc-labeled bone scintigraphy, using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), 99mTc-labeled pyrophosphate (PYP), or 99mTc-labeled hydroxymethylene diphosphonate (HMDP) as per clinical practice. Radiotracer uptake in the myocardium relative to bone was scored using the Perugini grading scale: grade 0 denotes no cardiac uptake; grade 1, mild uptake less than bone; grade 2, moderate uptake equal to bone; and grade 3, high uptake greater than bone.20 Scintigraphs considered positive (Perugini grade 1-3) by the local laboratory were centrally analyzed; centralized results were established as valid. Reviewers had access only to patients’ scintigraphy images but were not blinded to the purpose of the study. Recommendations at the time of the study also included serum and urine immunofixation electrophoresis and serum-free light chain assay.20,21
Patients with grade 1 cardiac uptake, in the absence of a confirmatory biopsy, were classified as inconclusive patients. Patients with grade 2 to 3 cardiac uptake and no monoclonal protein abnormalities were classified as having ATTR-CA.20,21 Patients with ATTR-CA with and without a pathogenic TTR gene variant were classified as having hereditary and wild-type ATTR-CA, respectively. Patients with grade 2 to 3 cardiac uptake and monoclonal protein abnormalities, in the absence of a confirmatory biopsy, were classified as inconclusive patients; when biopsy demonstrated TTR deposition, the diagnosis was ATTR-CA.
EndpointsThe primary endpoint was to assess the prevalence of ATTR-CA in patients with HFpEF and LVH ≥ 12mm. Secondary endpoints included assessing the prevalence of ATTR-CA by region, age, and type of ATTR-CA, and describing the characteristics of patients with ATTR-CA within the evaluated population.
Statistical analysisStatistical analyses were conducted in all patients who met the eligibility criteria. Prevalence was analyzed in patients who underwent both scintigraphy and hematologic tests. Patients without any of these tests were classified as not analyzable in terms of prevalence, but their demographics and baseline characteristics were collected.
Descriptive statistics were used to analyze the collected data. Missing data were excluded from the analyses. Qualitative variables are expressed as frequencies and compared using the Fisher exact test. Quantitative variables are expressed as mean (95% confidence interval) or median (with 25th to 75th interquartile range) and compared using ANOVA/Kruskal-Wallis test, depending on the distribution of the sample. Post-hoc analysis was performed using the Fisher, Student t-test, or Mann-Whitney U tests, as appropriate. Data were analyzed using SPSS (v18 or later). Statistical significance was defined as P<.05.
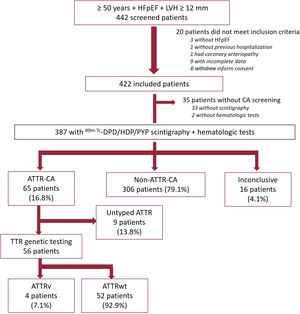
RESULTSDuring the study period, we included 442 patients with HFpEF from 20 hospitals (64% of the patients were included from cardiology departments and 27% from hospitals in which both cardiology and internal medicine participated). Of these, 20 patients were deemed ineligible according to the inclusion criteria (figure 1). Scintigraphy or hematologic tests were not performed in 35 patients who met the inclusion criteria and were assumed to be unscreened for CA. Therefore, 387 patients were considered analyzable in terms of ATTR-CA prevalence. A flow-chart of participants is shown in figure 1.
Study participants. ATTR, transthyretin amyloidosis; ATTR-CA, patients with confirmed diagnosis of ATTR-CA; ATTRv, hereditary transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; HFpEF, heart failure with preserved ejection fraction; LVH, left ventricular hypertrophy; Non-ATTR-CA, patients with HFpEF in which ATTR-CA was ruled out; TTR, transthyretin.
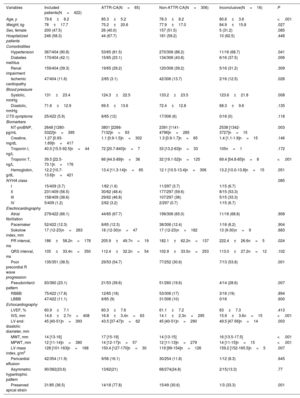
Among the 422 patients included, all but 4 were Caucasian (99.1%), 52.5% were men, and the mean age was 79.6 years. Most of the patients (58.3%) were evaluated during a HF hospitalization. Patients were most frequently included from hospitals in Catalonia (15.6%), the Basque Country (14.2%), Madrid (14.0%), and the Canary Islands (10.7%). The characteristics of the overall population are shown in table 1. Several patients had a history of hypertension, diabetes, and prior renal failure. NT-proBNP levels ranged from 1280 pg/L to 5322 pg/L [median, 2648 pg/L], and most of the patients were in New York Heart Association (NYHA) class I-II. Mean LVEF was 60.9±7.2%, and the median LV maximal wall thickness was 14mm [interquartile range, 13-16].
Clinical, analytical, electrocardiographic, and echocardiographic characteristics
| Variables | Included patients(N=422) | ATTR-CA(N=65) | Non-ATTR-CA(N=306) | Inconclusive(N=16) | P |
|---|---|---|---|---|---|
| Age, y | 79.6±8.2 | 85.3±5.2 | 78.3±8.2 | 80.6±3.6 | <.001 |
| Weight, kg | 78±17.7 | 75.2±20.6 | 77.9±17.0 | 84.9±15.8 | .027 |
| Sex, female | 200 (47.5) | 26 (40.0) | 157 (51.5) | 5 (31.2) | .085 |
| Hospitalized patients | 246 (58.3) | 44 (67.7) | 181 (59.2) | 10 (62.5) | .448 |
| Comorbidities | |||||
| Hypertension | 367/404 (90.8) | 53/65 (81.5) | 270/306 (88.2) | 11/16 (68.7) | .041 |
| Diabetes mellitus | 170/404 (42.1) | 15/65 (23.1) | 134/306 (43.8) | 6/16 (37.5) | .006 |
| Renal impairment | 159/404 (39.3) | 19/65 (29.2) | 120/306 (39.2) | 5/16 (31.2) | .309 |
| Ischemic cardiopathy | 47/404 (11.6) | 2/65 (3.1) | 42/306 (13.7) | 2/16 (12.5) | .028 |
| Blood pressure | |||||
| Systolic, mmHg | 131±23.4 | 124.3±22.5 | 133.2±23.5 | 123.6±21.8 | .008 |
| Diastolic, mmHg | 71.6±12.9 | 69.5±13.6 | 72.4±12.8 | 68.3±9.6 | .135 |
| CTS symptoms | 25/422 (5.9) | 8/65 (12) | 17/306 (6) | 0/16 (0) | .118 |
| Biomarkers | |||||
| NT-proBNP, pg/mL | 2648 [1280-5322]n=395 | 3801 [2266-7132]n=63 | 2391 [1141-4796]n=285 | 2538 [1342-3727]n=15 | .003 |
| Creatine, mg/dL | 1.27 [0.93-1.69]n=417 | 1.1 [0.9-1.5]n=302 | 1.3 [0.9-1.7]n=65 | 1.4 [1.1-1.9]n=15 | .146 |
| Troponin I, ng/L | 40.5 [15.5-92.5]n=44 | 72 [20.7-840]n=7 | 33 [13.2-63]n=33 | 105n=1 | .172 |
| Troponin T, ng/L | 39.5 [22.5-73.1]n=176 | 66 [44.5-89]n=36 | 32 [19.1-52]n=125 | 69.4 [54.8-85]n=8 | <.001 |
| Hemoglobin, g/dL | 12.2 [10.7-13.6]n=421 | 13.4 [11.3-14]n=65 | 12.1 [10.5-13.4]n=306 | 13.2 [10.0-13.8]n=15 | .051 |
| NYHA class | .085 | ||||
| I | 15/409 (3.7) | 1/62 (1.6) | 11/297 (3.7) | 1/15 (6.7) | |
| II | 231/409 (56.5) | 30/62 (48.4) | 177/297 (59.6) | 8/15 (53.3) | |
| III | 158/409 (38.6) | 29/62 (46.8) | 107/297 (36) | 5/15 (33.3) | |
| IV | 5/409 (1.2) | 2/62 (3.2) | 2/297 (0.7) | 1/15 (6.7) | |
| Electrocardiography | |||||
| Atrial fibrillation | 279/422 (66.1) | 44/65 (67.7) | 199/306 (65.0) | 11/16 (68.8) | .908 |
| Pacemaker | 52/422 (12.3) | 8/65 (12.3) | 38/306 (12.4) | 1/16 (6.2) | .904 |
| Sokolow index, mm | 17 (12-23)n=263 | 18 (12-30)n=47 | 17 (12-23)n=182 | 13 (9-30)n=9 | .683 |
| PR interval, ms | 186±58.2n=178 | 205.9±49.7n=19 | 182.1±62.2n=137 | 222.4±26.6n=5 | .024 |
| QRS interval, ms | 105±33.4n=350 | 112.4±32.2n=54 | 102.9±33.5n=253 | 113.0±27.2n=12 | .102 |
| Poor precordial R wave progression | 135/351 (38.5) | 29/53 (54.7) | 77/252 (30.6) | 7/13 (53.8) | .001 |
| Pseudoinfarct pattern | 83/360 (23.1) | 21/53 (39.6) | 51/260 (19.6) | 4/14 (28.6) | .007 |
| RBBB | 75/422 (17.8) | 12/65 (18) | 53/306 (17) | 3/16 (19) | .894 |
| LBBB | 47/422 (11.1) | 6/65 (9) | 31/306 (10) | 0/16 | .600 |
| Echocardiography | |||||
| LVEF, % | 60.9±7.1 | 60.3±7.6 | 61.1±7.2 | 63±7.3 | .413 |
| IVS, mm | 14.6±2.7n=408 | 16.8±3.4n=63 | 14.1±2.3n=295 | 15.9±3.6n=15 | <.001 |
| LV end-diastolic diameter, mm | 45 [40-51]n=393 | 43.5 [37-47]n=62 | 45 [40-51]n=290 | 49.5 [47-56]n=14 | .003 |
| MWT, mm | 14 [13-16] | 17 [15-19] | 14 [13-15] | 16 [13.5-17.5] | <.001 |
| MPWT, mm | 12 [11-14]n=380 | 14 [12-17]n=57 | 12 [11-13]n=279 | 14 [11-15]n=15 | <.001 |
| LV mass index, g/m2 | 128 [101-163]n=168 | 150.4 [127-170]n=30 | 119 [99-154]n=126 | 159.2 [152-165.5]n=5 | .007 |
| Pericardial effusion | 42/354 (11.9) | 9/56 (16.1) | 30/254 (11.8) | 1/12 (8.3) | .645 |
| Asymmetric hypertrophic pattern | 90/382(23.6) | 13/62(21) | 68/274(24.8) | 2/15(13.3) | .77 |
| Preserved apical strain | 31/85 (36.5) | 14/18 (77.8) | 15/49 (30.6) | 1/3 (33.3) | .001 |
ATTR-CA, transthyretin cardiac amyloidosis; CTS, carpal tunnel syndrome; IVS, interventricular septum; LBBB, left bundle branch block; LV, left ventricle; LVEF, left ventricle ejection fraction; MPVT, maximum posterior wall thickness; MWT, maximum wall thickness; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; RBBB, right bundle branch block; SD, standard deviation.
Data are expressed as No. (%), median [interquartile range], or mean±standard deviation.
When data were not available for all patients, N is indicated.
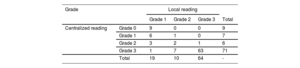
Scintigraphy was not performed in 33 included patients. All scintigraphs but 4 were performed with 99mTc-DPD. According to local center interpretation, 93 patients showed myocardial uptake (table 2). Among them, hematologic tests were performed in 89 out of 93 patients. In those without cardiac uptake, hematological tests were performed in 24 out of 296. The result was positive in 18 out of 113 (15.9%); only 3 biopsies were performed (1 TTR, 1 inconclusive and 1 compatible with myeloma). Scintigraphy central core reading reclassified 22 patients (23.6%), with a total of 84 patients exhibiting myocardial uptake (table 2).
Scintigraphy uptake according to local or central laboratory reading
| Grade | Local reading | ||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Total | ||
| Centralized reading | Grade 0 | 9 | 0 | 0 | 9 |
| Grade 1 | 6 | 1 | 0 | 7 | |
| Grade 2 | 3 | 2 | 1 | 6 | |
| Grade 3 | 1 | 7 | 63 | 71 | |
| Total | 19 | 10 | 64 | - | |
Only positive scintigraphs as per local review were centrally analyzed.
A total of 65 patients were confirmed to have ATTR-CA. ATTR-CA could not be confirmed or ruled out in 16 patients due to incomplete or absent histological analysis. For patients with both scintigraphy and hematologic tests, scenarios according to diagnostic test results and the final diagnosis are shown in figure 1 of the supplementary data.
The prevalence of ATTR-CA among patients with scintigraphy and hematologic tests was 16.8% (table 3). We found large differences among regions with a prevalence> 20% in the Canary Islands, Aragon, and the Basque Country (table 4). Prevalence increased with age, from 14.8% in patients aged 76 to 80 years to 28.6% in patients aged 86 to 90 years (table 5). None of the patients diagnosed with ATTR-CA were younger than 75 years. Genetic TTR testing was performed in 86% (56/65) of patients with a diagnosis of ATTR-CA, confirming ATTRwt in 52, and ATTRv in 4 (3 patients with p.Val122Ile and 1 with p.Val30Met).
Prevalence of ATTR-CA among patients with HFpEF and LVH ≥ 12mm
| Variable | No. | Included patients (%) | Analyzable patients (%) |
|---|---|---|---|
| ATTR-CA | 65 | 15.4 | 16.8 |
| Non-ATTR-CA | 306 | 72.5 | 79.1 |
| Inconclusive | 16 | 3.8 | 4.1 |
| Not analyzable | 35 | 8.3 | - |
| Total | 422 | 100.0 | 100.0 |
ATTR-CA, transthyretin cardiac amyloidosis; HFpEF, heart failure with preserved ejection fraction; LVH, left ventricular hypertrophy.
ATTR-CA prevalence per region of residence
| Geographical region | Included patients | Patients with ATTR-CA | Patients with ATTR-CA per region (%) |
|---|---|---|---|
| Basque Country | 59 | 13 | 22.03 |
| Catalonia | 59 | 7 | 11.86 |
| Community of Madrid | 58 | 9 | 15.52 |
| Canary Islands | 45 | 17 | 37.78 |
| Aragon | 37 | 9 | 24.32 |
| Andalusia | 24 | 2 | 8.33 |
| Galicia | 22 | 1 | 4.55 |
ATTR-CA, transthyretin cardiac amyloidosis.
ATTR-CA prevalence per age group
| Variable | 50-75 y | 76-80 y | 81-85 y | 86-90 y | ≥ 91 y |
|---|---|---|---|---|---|
| ATTR-CA | 0 (0) | 12 (14.8) | 21 (18.6) | 20 (28.6) | 12 (63.2) |
| Non-ATTR-CA | 102 (97.9) | 64 (79.0) | 84 (74.3) | 49 (70.0) | 7 (36.8) |
| Inconclusive | 2 (1.9) | 5 (6.2) | 8 (7.1) | 1 (1.4) | 0 (0.0) |
| Total | 104 | 81 | 113 | 70 | 19 |
ATTR-CA, transthyretin cardiac amyloidosis.
The data are expressed as No. (%).
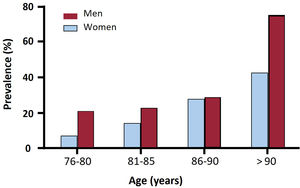
There was a trend toward a higher prevalence of ATTR-CA among men (19.7% vs 13.8% in men and women, respectively, P=.085), without significant differences between sexes in any age range (figure 2). The prevalence of ATTRv was 13% (3/23) in women and 3% (1/33) in men with ATTR-CA who underwent genetic testing.
Clinical characteristics of patients with ATTR-CAClinical, analytical, electrocardiographic, and echocardiographic characteristics of patients with ATTR-CA compared with those without ATTR-CA and inconclusive patients are presented in table 1. Not unexpectedly, patients with ATTR-CA were older than patients without ATTR-CA (P <.001). Compared with patients without ATTR-CA, those with ATTR-CA had higher median NT-proBNP and Troponin T levels (P=.001 and P <.001, respectively). Half of the patients with ATTR-CA were in NYHA class I-II. Although no statistically significant differences were found, a higher proportion of patients with ATTR-CA were in NYHA class III than in the other groups (46.8% vs 36.0% vs 33.3%; P=.085). Patients with ATTR-CA had lower systolic blood pressure than patients without ATTR-CA (P=.005). Most electrocardiographic features were similar among groups, although patients with ATTR-CA had a significantly higher prevalence of poor precordial R wave progression (P=.001) and pseudoinfarct pattern (P=.004) than patients without ATTR-CA.
Patients with ATTR-CA had moderately increased LV wall thickness, which was significantly higher than that in patients without ATTR-CA (P <.001). Additionally, the LV mass index was significantly increased in patients with ATTR-CA than in patients in whom ATTR-CA was excluded (P=.008). In patients with ATTR-CA, LV end-diastolic diameter was significantly lower than in the other groups (P=.003).
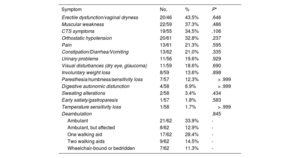
ATTR-CM patients were further evaluated to identify any ATTR-related extracardiac symptoms (table 6). No statistically significant differences were found between groups.
ATTR symptoms within confirmed patients with ATTR-CA
| Symptom | No. | % | P* |
|---|---|---|---|
| Erectile dysfunction/vaginal dryness | 20/46 | 43.5% | .646 |
| Muscular weakness | 22/59 | 37.3% | .486 |
| CTS symptoms | 19/55 | 34.5% | .106 |
| Orthostatic hypotension | 20/61 | 32.8% | .237 |
| Pain | 13/61 | 21.3% | .595 |
| Constipation/Diarrhea/Vomiting | 13/62 | 21.0% | .335 |
| Urinary problems | 11/56 | 19.6% | .929 |
| Visual disturbances (dry eye, glaucoma) | 11/59 | 18.6% | .690 |
| Involuntary weight loss | 8/59 | 13.6% | .898 |
| Paresthesia/numbness/sensitivity loss | 7/57 | 12.3% | > .999 |
| Digestive autonomic disfunction | 4/58 | 6.9% | > .999 |
| Sweating alterations | 2/58 | 3.4% | .434 |
| Early satiety/gastroparesis | 1/57 | 1.8% | .583 |
| Temperature sensitivity loss | 1/58 | 1.7% | > .999 |
| Deambulation | .845 | ||
| Ambulant | 21/62 | 33.9% | - |
| Ambulant, but affected | 8/62 | 12.9% | - |
| One walking aid | 17/62 | 28.4% | - |
| Two walking aids | 9/62 | 14.5% | - |
| Wheelchair-bound or bedridden | 7/62 | 11.3% | - |
CTS, carpal tunnel syndrome.
There were no significant differences between men and women with ATTR-CA in terms of age (85.1±5.9 vs 85.7±3.9 years, P=.653). The prevalence of hypertension was significantly higher in women (100% vs 74.4%; P=.004), with a higher number of women being in NYHA III (64.0% vs 35.1%, P=.017). Women also had higher diastolic blood pressure (75.8±15.8 vs 65.3±10.1mmHg, P=.009).
DISCUSSIONThis study elucidates the prevalence of ATTR-CA among patients with HFpEF in Spain (figure 3). The high prevalence of ATTR-CA in this population highlights the importance of awareness of this disease among HF specialists, as specific treatment is available. Ours is the largest multicenter study conducted to date in patients with HFpEF.
Prevalence of ATTR-CA among HFpEF patientsThe precise prevalence of ATTR-CA is still unknown, but various studies have attempted to elucidate its frequency in different HF populations (table 2 of the supplementary data).7,12–15,17,18,21–25 So far, 7 studies—all but 2 of which were single-center studies—have investigated the prevalence of ATTR-CA among HFpEF patients.13–17,23,25 The 2 larger studies reported prevalence rates ranging from 6.3% to 13.3% (n=286; n=120).14,16 Our multicenter study identified a prevalence of 16.8% among a larger cohort (n=422; 387 screened for CA). Recently, a multicenter study conducted in Spain with 453 patients, all aged ≥ 65 years with any form of HF and LVH> 12mm evaluated in internal medicine departments between February 2020 and March 2021,18 reported a prevalence of 16.8%. Of these patients, 76.6% with ATTR-CA had an LVEF> 50%. However, the prevalence of patients with ATTR-CA among the HFpEF subpopulation was not reported in that study.18
Despite previously published differences between HF patients admitted to internal medicine vs cardiology departments,26 and variations in the populations included in the study by Ruiz-Hueso et al.18 and our own, the prevalences found in these studies were similar. Notably, the rate of ATTR genetic testing was 86% in our study compared with 47% in the study by Ruiz-Hueso et al.18 As the inclusion dates were similar, these differences might be due to greater awareness of the disease at the time or to more routine genetic testing for hereditary cardiomyopathies among cardiologists, who were the main recruiters in our study. We found a 7.1% prevalence of ATTRv among older patients with ATTR-CA (> 75 years), highlighting the need to perform genetic testing regardless of age.
With the largest cohort to date, our study provides evidence that ATTR-CA is a frequent cause of HFpEF in older adults. This supports the recommendation of the 2021 ESC Guidelines to screen for the presence of CA in the differential diagnosis of HFpEF.11
ATTR-CA in womenAlthough ATTR-CA has been classically described as a disease affecting older men,27,28 40% of the patients with ATTR-CA identified in our study were women. In line with our observations, previous studies (most of them carried out in Spain) also reported a high percentage of women among the patients diagnosed with ATTR-CA (33%-50%).13,16–18 On the other hand, other studies have found that 90% to 100% of patients with ATTR-CA were men.7,12,15,22 HFpEF has been reported to be more common among women, who tend to live longer, making it intriguing that most ATTR-CA studies report a male preponderance in the disease.7,12,14,15,22,23 A plausible explanation is that ATTR-CA is more commonly suspected in men than in women, possibly due to unconscious gender inequalities embedded in some health care systems.29 It has recently been suggested that nonindexed wall thickness measurements may have contributed to both underrepresentation and delays in the diagnosis of ATTR-CA in affected women.30,31 Although women were diagnosed similarly to men in our study, the proportion of women with ATTR-CA in NYHA class III was significantly higher than that in men (64% vs 35%), which might indicate a delayed diagnosis.
Only 2 previous studies have described the prevalence of ATTR-CA by sex,14,18 with significant differences observed only by AbouEzzeddine et al.14 We found a higher prevalence of ATTR-CA in men than in women (19.7% vs 13.8%, respectively), but this difference was not statistically significant (P=.085). Although the possibility of a lack of statistical power cannot be excluded, the number of patients included in our study was higher than in the study by AbouEzzeddine et al.14 Interestingly, we found a higher prevalence of ATTRv among women than among men (13% vs 3%), reinforcing sex as a predictor of ATTRv in older patients with ATTR-CA.32
Our study highlights the need to raise suspicion of ATTR-CA among older patients with HFpEF and LVH independently of sex.
Implications of correct ATTR-CA screening and diagnosisImportantly, almost 25% of positive cardiac scintigraphs (Perugini grades 1-3) were reclassified upon central core blinded reading. Four patients who were initially classified as grade 1 were finally considered as grade 2/3, leading to the diagnosis of 3 patients with ATTR-CA after excluding monoclonal protein. The remaining patient exhibited monoclonal protein, and a subsequent cutaneous biopsy did not elucidate the diagnosis. As correct cardiac scintigraphy interpretation can directly impact the final diagnosis and/or the need for invasive evaluation,2 our results underscore the central role of nuclear medicine in ATTR-CA diagnosis and the need to integrate scintigraphy results with suggestive findings on echocardiography or cardiac magnetic resonance for a noninvasive ATTR-CA diagnosis. Although high intra- and interobserver reproducibility for visual scoring has been documented,33 misleading classifications can still occur.1,34 In this regard, single photon emission computed tomography (SPECT) imaging is particularly useful in positive or equivocal cases to differentiate myocardial from blood pool signal and to describe regional heterogeneity.35
Data from the National Amyloidosis Center in the United Kingdom reported that, in the 3 years prior to diagnosis, patients had up to 27 hospital visits and 5 hospitalizations.36 Given their median NT-proBNP levels, mean interventricular septum thickness, history of hospitalization for HF, and the fact that half were included during hospitalization and half were already in NYHA class III-IV, it would appear that diagnosis often occurs late in the disease course. Whether ATTR-CA could have been suspected and diagnosed earlier in our study is unknown, but the data might suggest missed opportunities for diagnosis. As previously mentioned, we found almost no differences in ATTR-related symptoms in patients with ATTR-CA compared with those without ATTR-CA, indicating a high clinical overlap. The nonspecificity of symptoms in ATTR is known to contribute to its delayed diagnosis.
The ESC position statement recommends screening for CA in all patients with LVH ≥ 12mm and at least 1 additional red flag.2 As this would require a large number of tests that might yield negative results, several scoring systems are under development. These scores aim to improve suspicion of ATTR-CA and help prioritize which patients should be tested for the disease.37,38 In both scores, age is one of the variables contributing to a higher risk of amyloidosis. This support our findings and those of previous studies, showing that the prevalence of ATTR-CA increases with age14,18,25 and that no patients are diagnosed before the age of 75 years.18
Correct and timely diagnosis of ATTR-CA has important implications for patients. It modifies symptomatic treatment compared with patients without ATTR-CA, as commonly used medications in HF, such as ACEI, ARNi and beta-blockers, are frequently poorly tolerated by patients with ATTR-CA, despite controversy surrounding the use of beta-blockers.2,39,40 In addition, disease-modifying-treatment should be initiated as early as possible to halt amyloid deposition. The 2021 ESC guidelines recommend tafamidis in patients with HF and amyloidosis, in ATTRwt and ATTRv patients and NYHA class I or II to reduce symptoms, CV hospitalization, and mortality (class and level IB).11 The decision to start disease-modifying treatment depends on whether the patient will meaningfully benefit.41
Furthermore, diagnosing ATTR-CA is the first step toward performing genetic testing to identify patients with ATTRv, allowing the proper identification of relatives at risk of developing the disease or who may already be affected by the condition. Several treatments are available for ATTRv patients, depending on their phenotype.42 In our study, we found that 7.1% of patients with ATTR-CA had ATTRv, but we did not evaluate the impact of treatment as it has been recently described.32
Strengths and limitationsThe main strength of this study is its prospective, multicenter design, which includes a large number of patients with HFpEF from nearly all regions in Spain, aged 50 years and older, with LVH ≥ 12mm, as currently recommended. Furthermore, both ambulatory and hospitalized patients were included, reinforcing the need for suspicion in both scenarios. The characteristics of patients with ATTR-CA described in this study are representative of real-life patients. Additionally, scintigraphy images were centrally analyzed, avoiding inter-center bias.
However, limitations must be acknowledged. This study has the inherent limitations of an observational study, including potential selection bias. The number of patients presenting to each study center or referred from other practices, and details related to preliminary screening elsewhere, were not collected. Although the study protocol stipulated the enrollment of consecutive patients who satisfied eligibility criteria, some variability in enrollment practices likely occurred. The inclusion of only patients with a previous history of HF hospitalization might have influenced the observed prevalence of the disease.
Although both serum-free light chain quantification and serum and urine immunofixation are necessary to exclude AL amyloidosis,43 we found that 23.4% of hematologic tests were incomplete. Biopsy was not conducted in most patients requiring histological confirmation. Consequently, incomplete testing or inconclusive results in some cases may have led to an overestimation of the reported prevalence of ATTR-CA in our study. Additionally, since only positive scans (grades 1-3) in scintigraphy were centrally analyzed, this could introduce some bias. Another limitation is that we did not collect specific data on the use of SPECT, as there were no strong recommendations regarding its use at the time of the study, and we cannot confirm whether it was used.
Additionally, we found some limitations in patient inclusion and data acquisition in the study period due to the impact of the COVID-19 pandemic.
CONCLUSIONSThis nationwide multicenter prevalence study found that the prevalence of ATTR-CA in patients with HFpEF and LVH ≥ 12mm was 16.8%. We report an increasing prevalence of ATTR-CA with age, with no patient diagnosed aged <75 years. Moreover, our study did not detect significant differences between sexes. Our findings highlight the need for more intensive ATTR-CA screening among at-risk patients with HFpEF to allow previously undiagnosed patients earlier access to disease-modifying therapies.
FUNDINGThe study was sponsored by Pfizer SLU. Pfizer personal took part in the design of the study and writing of the article.
ETHICAL CONSIDERATIONSThe study protocol, amendments, and informed consent were reviewed and approved by an independent ethics committee from each participating center. All patients provided written informed consent before study enrollment. Possible sex/gender biases have been taken into account in the preparation of this paper. Both men and women with HFpEF were included in the study; 52.2% were men and 47.5% were women.
STATEMENT ON THE USE OF ARTIFICAL INTELLIGENCENo artificial intelligence tool was used to prepare this article.
AUTHORS’ CONTRIBUTIONSP. García-Pavía, J.M. García-Pinilla, and P. Tarilonte contributed with the conception and design of the study. P. García-Pavía, J.M. García-Pinilla, A. Lozano-Bahamonde, S. Yun, A. García-Quintana, J.J. Gavira-Gómez, M.A. Aibar-Arregui, G. Barge-Caballero, and J. Núñez-Villota conducted the research. P. García-Pavía, J.M. García-Pinilla, and L. Bernal drafted and wrote the first version of the manuscript. A. Lozano-Bahamonde, S. Yun, A. García-Quintana, J.J. Gavira-Gómez, M.A. Aibar-Arregui, G. Barge-Caballero, J. Núñez Villota, and P. Tarilonte critically revised drafts and the final manuscript. All authors provided final approval to the manuscript.
CONFLICTS OF INTERESTThe study is sponsored by Pfizer SLU. L. Bernal and P. Tarilonte are full-time employees of Pfizer SLU. P. Tarilonte holds Pfizer stocks and stock options. P. García-Pavía reports speaking fees from Pfizer, Bridgebio, Ionis Pharmaceuticals, AstraZeneca, Novo Nordisk, Intellia, and Alnylam Pharmaceuticals; consulting fees from Pfizer, Bridgebio, Neuroimmune, Alnylam Pharmaceuticals, AstraZeneca, Novo Nordisk, ATTRalus, Intellia, General Electric, and Alexion; and research/educational support to his institution from Pfizer, Bridgebio, Novo Nordisk, AstraZeneca, Intellia, and Alnylam Pharmaceuticals. J.M. García-Pinilla reports speaking fees from Pfizer, AstraZeneca, Alnylam Pharmaceuticals, Boehringer Ingelheim, Abbott, Medtronic, Impulse Dynamics, Novartis, Rovi, and Orion Pharma; consulting fees from Pfizer, Alnylam Pharmaceuticals, and Bristol-Myers Squibb; and research support to his institution from Novartis. P. García-Pavía and J.M. García-Pinilla are coordinators of the study and have received fees for this work. A. Lozano-Bahamonde declares no conflicts of interest. S. Yun has served as a speaker at scientific meetings for AstraZeneca, Bristol-Myers Squibb, Rovi, and Pfizer; received funding from Bristol-Myers Squibb, Rovi, and Pfizer for scientific meeting expenses; and his institution has received research grants/educational support from AstraZeneca, Novartis, Vifor, and Pfizer. A. García-Quintana reports speaking fees from Pfizer, AstraZeneca, Alnylam Pharmaceuticals, Boehringer Ingelheim, Medtronic, Novartis, Rovi, Novo Nordisk, and Bayer; and consulting fees from Bayer, Novartis, and Novo Nordisk. J.J. Gavira-Gómez declares no conflicts of interest. M.A. Aibar-Arregui reports speaking fees from Pfizer and Alnylam, and has received research grants/educational support from Pfizer, Alnylam, and Akcea. G. Barge-Caballero reports consulting fees from Pfizer, AstraZeneca, and Boehringer Ingelheim; speaking fees from Pfizer, AstraZeneca, and Boehringer Ingelheim; and has received research grants/educational support from Pfizer. J. Núñez-Villota reports personal fees or advisory board memberships from Alleviant, AstraZeneca, Boehringer Ingelheim, Bayer, Novartis, Novo Nordisk, Pfizer, Rovi, and Vifor Pharma (outside the submitted work).
P. García-Pavía is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial processing of the manuscript has been followed.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
- •
ATTR-CA has become the most commonly diagnosed type of cardiac amyloidosis, although its prevalence remains uncertain. While ATTR-CA is a frequent cause of HFpEF, with a prevalence ranging from 5% to 20% in this population, systemic screening is not yet consistently implemented in clinical practice. To date, no national multicenter studies have explored the prevalence of ATTR-CA among HFpEF patients.
- •
This is the first multicenter nationwide study evaluating the prevalence of ATTR-CA in patients with HFpEF and LVH, mostly enrolled from cardiology departments, using the current definition of preserved ejection fraction (≥ 50%) and the recommended LV wall thickness threshold for CA suspicion (≥ 12mm). We found a prevalence of 16.8%, indicating that ATTR-CA is a significant contributor to HFpEF in both men and women aged 75 years and older. These results highlight the need for more intensive ATTR-CA screening in this population.
We would like to thank all the investigators of the PRACTICA study.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2024.07.005