Premature ventricular complexes (PVCs) in the absence of known structural heart disease used to be considered a benign entity. Early studies supporting this conclusion were limited by small sample sizes and limited cardiac testing during follow-up. More recent work has revealed the long-term effects of frequent PVCs and their ability to cause or contribute to cardiomyopathy and heart failure symptoms. In this editorial, we review the risk factors associated with development of PVC-induced cardiomyopathy. We also review the available treatment options focusing on catheter ablation and discuss the overall risks and effectiveness of ablation and medical management. Ultimately, we hope to provide a framework for the management and follow-up of patients with PVC-induced cardiomyopathy or at risk for developing this condition.
PREVALENCE AND PROGNOSISThe prevalence of PVCs depends on the comorbidities of the patients being screened and the duration of monitoring. In the general population, it is estimated that more than 60 PVCs/h are seen in about 1% to 4% of the general population.1 The prognosis of frequent PVCs depends on the underlying cardiac substrate. In patients with underlying cardiac disease such as previous myocardial infarction, high frequency PVCs have long been known to adversely impact on prognosis.2
For years, patients with frequent PVCs but no underlying cardiac disease were considered to have a benign prognosis. This conclusion, however, was mainly based on a study with a small sample size and limited diagnostic testing.1 More recent studies have clearly shown the potential detrimental effects of frequent PVCs in patients with apparently normal hearts.
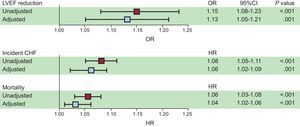
The development and reversibility of PVC-induced cardiomyopathy has been clearly established. While there are multiple predictors of PVC-induced cardiomyopathy, patients with very frequent PVCs appear to be at a particular risk, with 1 study demonstrating a cutoff PVC burden of 24% with the highest sensitivity and specificity in predicting development of cardiomyopathy.3 More recent studies have shown that increased long-term cardiac risk may be predicted by even lower levels of PVCs. A recent study by Dukes et al.,4 showed that patients within the highest PVC burden quartile (0.123%-17.7%) had a higher risk of decreased left ventricular ejection fraction (EF), development of congestive heart failure, and mortality compared with patients within the lowest PVC burden quartile (0%-0.002%) during a median follow-up > 13 years (Figure 1). In that study, heart failure was correlated with a much lower PVC burden than the burden traditionally associated with PVC-induced cardiomyopathy. The causative or modifiable role of PVCs in this sample was not established and further long-term studies will be needed to confirm whether such a low PVC burden can lead to PVC-induced cardiomyopathy.
Squares represent unadjusted (red) and adjusted (blue) odds ratios (for log base 2-transformed percent of premature ventricular contractions as a predictor of any reduction in qualitative left ventricular ejection fraction between the baseline and 5-year echocardiograms or hazard ratios for incident congestive heart failure and mortality. Multivariable models included adjustment for age, sex, race, body mass index, and a history of hypertension, diabetes, coronary artery disease, beta-blocker use, Holter-based atrial fibrillation, and number of Holter-based ventricular tachycardia episodes. Error bars indicate 95% confidence intervals. Hazard ratios express the increase in risk per doubling of the percent of premature ventricular contractions. Reproduced with permission from Dukes et al.4 95% CI, 95% confidence interval; CHF, congestive heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; OR, odds ratio; PVC, premature ventricular contraction.
Among the identified risk factors for the development of PVC-Induced cardiomyopathy are high-frequency PVCs, longer duration of PVCs, epicardial or broad QRS complex PVCs, interpolated PVCs, male sex, lack of short-term variability of the PVC burden, and PVCs in asymptomatic patients.
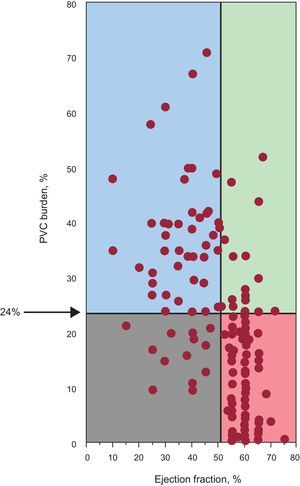
While no exact PVC frequency separates those at risk for or those safe from PVC-induced cardiomyopathy, a prior study including patients referred for PVC ablation reported a 24% PVC burden to have the best sensitivity and specificity in predicting the development of cardiomyopathy (Figure 2).3 Importantly, however, patients with a PVC burden of 10% or lower can have a reversible PVC-induced cardiomyopathy and those with a PVC burden approaching 50% can have preserved cardiac function.
Scattergram indicating the relationship between premature ventricular contraction burden and ejection fraction. Reproduced with permission from Baman et al.3 PVC, premature ventricular contraction.
The duration of PVCs can often be challenging to ascertain due to the often subtle onset of symptoms or asymptomatic status. Longer durations of frequent PVCs have been associated with a higher prevalence of cardiomyopathy. An asymptomatic status is also associated with cardiomyopathy and may reflect a longer exposure time in these patients.5 The development of cardiomyopathy is generally a slow process over several months or years rather than weeks. This has important implications for the management and follow-up of patients with high PVC burdens. A low PVC variability over a 24-hour recording period has recently been shown to predict the development of PVC-induced cardiomyopathy independent of other factors.6
While originally PVCs from the right ventricular outflow tract were thought to primarily be responsible for the development of PVC-induced cardiomyopathy, more recent studies have shown that an epicardial origin portends the highest risk of cardiomyopathy. Broader QRS complexes during PVCs is also an independent risk factor for the development of cardiomyopathy.7 A possible mechanistic explanation is that broad QRS complexes and epicardial origin may represent a greater degree of mechanical ventricular dyssynchrony during PVCs and may have more deleterious effects on ventricular chamber function and size. While the mechanism is not entirely clear, interpolated PVCs also convey a greater risk of developing cardiomyopathy.8 Analysis of further data on larger cohorts will likely identify other risk factors for the development of PVC-induced cardiomyopathy. A recent large scale study showed that among patients referred for catheter ablation, male sex may be an independent risk factor for the development of cardiomyopathy.9
MECHANISMS OF PREMATURE VENTRICULAR COMPLEX-INDUCED CARDIOMYOPATHYThe mechanisms behind PVC-induced cardiomyopathy are not entirely clear. Early descriptions referred to PVC-induced cardiomyopathy as a tachycardia-mediated cardiomyopathy; however, this explanation is not likely to be valid as many such patients have average or even low heart rates. A more likely explanation is abnormal ventricular activation resulting in mechanical dyssynchrony. In support of this theory is the higher frequency of PVC-induced cardiomyopathy in patients with PVCs with wider QRS duration and epicardial location. Unlike in some animal models,10 the time-course of development of PVC-induced cardiomyopathy in humans is much longer.5
It is important to recognize that in patients with other underlying heart disease, frequent PVCs can result in further impairment of cardiac function. In patients whose cardiac function is out of proportion to the underlying cardiac process (ie, limited extent of coronary artery disease or minimal myocardial scarring) and with frequent PVCs, eliminating the PVCs can often lead to improvement, if not normalization, of cardiac function.11,12
PATIENT PRESENTATIONPatients with frequent PVCs most often present in 1 of 3 categories: asymptomatic, symptomatic acutely from individual PVCs, or symptomatic due to the cumulative hemodynamic effects of the PVCs.
Asymptomatic patients often present after incidental discovery on routine ECG or physical examination. Presentation in these patients can often be late and an asymptomatic status is a risk factor for PVC-induced cardiomyopathy.5
Symptoms due to the acute effects from individual PVCs include but are not limited to palpitations, chest discomfort, or shortness of breath. Symptoms may be secondary to the actual PVCs or the hypercontractile beats that may succeed compensatory pauses that often follow PVCs. Symptoms due to the cumulative effect of frequent PVCs can range from mild fatigue to decompensated heart failure requiring aggressive therapy.
Rarely, patients may present with sudden cardiac death, and the presence of frequent PVCs in these patients should prompt thorough investigation into occult structural heart disease. Another presenting scenario is in patients with an existing biventricular pacing device in whom frequent PVCs can lead to suboptimal resynchronization benefit. In these patients, the role of PVCs in the initial development of cardiomyopathy should be considered.
Indications to initiate therapy to suppress PVCs include bothersome symptoms, the presence of cardiomyopathy (decreased EF or ventricular dilatation), PVCs triggering malignant ventricular arrhythmias, and PVCs limiting optimal biventricular pacing. Whether patients with no symptoms or evidence of cardiomyopathy but with very frequent PVCs (> 20%) should be treated with either medications or ablation to prevent future risk of cardiomyopathy vs repeat assessment of left ventricular function remains to be determined. In this article we will focus on patients who present with or are at risk for developing PVC-induced cardiomyopathy.
WORK-UP AND MANAGEMENTThe initial work-up in a patient presenting with frequent PVCs should evaluate for other underlying structural heart disease with an echocardiogram or stress test. A 12-lead Holter monitor can quantify the PVC burden and assess for the presence of a predominant PVC. Cardiac magnetic resonance imaging with gadolinium is particularly helpful in determining the presence of scar, which may prompt further evaluation.
A thorough search for secondary causes of PVCs should be performed and managed when present. Potential culprits include other metabolic disorders (ie, hyperthyroidism), caffeine intake, intake of stimulants, or physical or emotional stressors. While behavioral changes (ie, decreased caffeine, alcohol, or tobacco intake) are unlikely to have a significant impact in decreasing PVC burden,13 they are still reasonable initial strategies given their low risk and potential secondary benefit.
The slowly developing and frequently reversible process of PVC-induced cardiomyopathy allows physicians to consider various modes of therapy. In general, options include management of secondary causes, pharmacotherapy aimed to suppress PVCs, or catheter ablation to reduce or eliminate PVCs.
Medical ManagementPharmacotherapy to suppress PVCs includes beta-blockers, calcium channel blockers, and other antiarrhythmic drugs. Results of the use of beta-blockers and calcium channel blockers are modest with reported efficacy rates in the 20% range. Use of these drugs may also be limited by the development of fatigue or worsening bradycardia. Nevertheless, these are reasonable first-line options due to their relatively low adverse effect profile and potential secondary benefit, particularly with beta-blockers in the setting of cardiomyopathy. Symptomatic benefit may also be present without appreciable decreases in the PVC burden with dampening of the contractile force of the PVCs or the often hypercontractile beats following PVCs.
Several antiarrhythmic drugs for the treatment of PVCs are not recommended in the presence of cardiomyopathy. Among the antiarrhythmic medications available, the most widely studied is amiodarone. In the Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure (CHF-STAT), 674 patients with cardiomyopathy and PVCs were randomized to amiodarone vs placebo. At 3 months, amiodarone decreased hourly PVCs (44±145 vs 254±370, P<.001) with 69% of patients experiencing an 80% decrease in PVC burden. There was also an overall improvement in EF at 6 months (33.7±11.0% vs 24.9±8.3%, P<.001).14 While amiodarone may be effective in reducing the PVC burden in most patients and has shown some benefit in improving cardiomyopathy in patients with PVCs, its long-term use is limited by its adverse effect profile. In the above-mentioned study, 27% discontinued the medication due to adverse effects.
Catheter AblationOver the past decade, catheter ablation has emerged as a relatively safe and effective option to pharmacotherapy for PVC elimination. In patients with PVC-induced cardiomyopathy, successful elimination of PVCs with ablation frequently restores ventricular function. Radiofrequency-catheter ablation has been shown to be superior to pharmacotherapy in patients with right ventricular outflow tract PVCs.15
A recent multicenter analysis of 1185 patients undergoing catheter ablation for idiopathic PVCs reported an overall acute procedural success rate of PVC elimination of 84%. Predictors of success were right ventricular outflow tract PVC location and monomorphic as opposed to multiple PVC morphologies. Long-term maintenance of at least 80% PVC reduction was maintained at follow-up of antiarrhythmic medications in 71% of patients. In this study, among the 245 patients with PVC-induced cardiomyopathy, the mean EF improved from 38% to 50% (P<.01). Major complications were reported in 2.4% of patients with no procedure related mortality.9
Multiple studies have confirmed the benefit of ablation in patients with PVC-induced cardiomyopathy with normalization of EF often being reported in > 80% of patients undergoing successful ablation (Figure 3).16 Some patients with severely decreased left ventricular function secondary to frequent PVCs who qualify for defibrillator implantation based on EF may no longer meet the criteria following successful ablation.
(A) Patients with premature ventricular contraction-induced cardiomyopathy who underwent a successful catheter ablation experienced a significant improvement of ejection fraction compared with (B) a control group without ablation who experienced no improvement in ejection fraction after 19 months. Reproduced with permission from Bogun et al.16
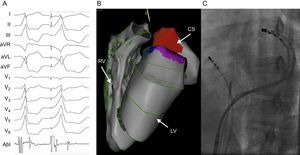
With improvements in mapping and ablation technology, the role of catheter ablation to eliminate PVCs is expanding. Challenging PVC locations such as the papillary muscles and epicardium are now more readily accessible (Figure 4). Given its higher efficacy over pharmacotherapy, relatively low procedural risk, and the low rate of spontaneous PVC resolution, catheter ablation is currently being evaluated as a potential first line therapy in patients with PVC-induced cardiomyopathy.
Patient with frequent epicardial premature ventricular contractions successfully ablated from the coronary sinus. A: Clinical premature ventricular contraction followed by a reasonable pace match from within the coronary sinus. Note the early activation time on the ablation catheter preceding the spontaneous premature ventricular contraction when positioned in the distal coronary sinus. B: 3-dimensional electroanatomic map using the Carto (Biosense Webster) mapping system. The area shaded red in the coronary sinus represents sites of early premature ventricular contraction activation. C: Fluoroscopic image of the ablation catheter in the coronary sinus. A simultaneous angiogram was also taken to ensure adequate distance between the ablation catheter and the coronary arteries. Elimination of PVCs in this patient resulted in normalization of cardiac function and chamber sizes. Abl, ablation; CS, coronary sinus; LV, left ventricle; PVC, premature ventricular contraction; RV, right ventricle.
When considering catheter ablation as a treatment strategy, one must weigh the likelihood of success and clinical improvement vs the potential risk. Factors to evaluate include PVC frequency, anticipated PVC location, the number of PVC morphologies, pharmaceutical alternatives, and patient age and comorbidities. Patients with frequent monomorphic PVCs from the right ventricular outflow tract appear to have the highest rates of success with ablation while those with multifocal and/or epicardial PVCs appear to have the lowest success rates.9
SUMMARY AND RECOMMENDATIONSExisting guidelines on the management of patients with frequent PVCs inadequately reflect currently available data. The harm associated with frequent PVCs in patients both with or without underlying cardiac disease has been increasingly recognized. Moreover, in the case of PVC-induced cardiomyopathy, PVC elimination has a clearly demonstrated clinical benefit.
Frequent PVCs have potential deleterious effects in both patients with or without underlying cardiac disease. Initial work-up for all patients with frequent PVCs should include an objective PVC burden assessment and an assessment of cardiac function. Patients with cardiac arrest or other high-risk symptoms should undergo more detailed investigation for occult cardiac disease. Indications for PVC suppression or elimination include limiting symptoms, evidence of PVC-induced cardiomyopathy, PVC triggered ventricular tachyarrhythmias, and limitation of biventricular pacing.
Antiarrhythmic drugs can eliminate PVCs in some patients but the benefits of such an approach must be weighed against the risk of long-term antiarrhythmic use. When successful, catheter ablation offers a high likelihood of resolving PVC-induced cardiomyopathy and should be strongly considered, particularly in patients with monomorphic and nonepicardial PVCs.
Management in patients with very frequent PVCs without symptoms or evidence of cardiac dysfunction may include PVC suppression vs repeat assessment of left ventricular function.
CONFLICTS OF INTERESTNone declared.