The vast majority of developed countries provide access to health services on the basis of clinical and health needs, and thus have chosen to fund these services publicly. This approach, apparently rooted in values of equity in health care, can also be explained in terms of efficiency. If the aim is to obtain the maximum amount and quality of life using the resources available, then extravagance has to be renounced in order to focus on what is clinically effective. Personal preferences can always be satisfied individually according to the ability and willingness to pay for them.
However, the effectiveness criterion is insufficient when the way innovation and aging is approached continues to modulate the growth rate of the percentage of a country's wealth that is dedicated to health services. Social costs have to be taken into account in order to choose the least expensive services from among the effective ones. This is not simply a question of saving money, but of preventing unnecessary deaths and suffering. For example, if the cost of lapatinib + capecitabine, a second-line treatment for breast cancer, is €732 000 per year in Spain (€1.5-€2 million per quality-adjusted life-year [QALY])1, the problem is not that €2 million is a lot of money, but that the value of the best alternative foregone (opportunity cost) by using these recourses is between 150 QALY and 200 QALY. As Salvador Espriu wrote, “sometimes it is necessary and right for a man to die for a country, but an entire country should never die for one man.” The 150 QALY to 200 QALY are lost simply by assuming that the best alternative foregone is approximately the average cost of obtaining 1 QALY in Spain2 or the United Kingdom.3 In the setting of clinical practice, Cochrane wrote long ago that individual patients should not be treated to the limit of the impossible because, as Donabedian stated, efficiency is the hallmark of virtuosity in medicine.
In Spain and throughout Southern Europe, the current economic situation strengthens the call for sensible policies. Data from the Ministry of Health show that between 2009 and 2013 public health spending decreased by 13%; despite this reduction in expenditure, there is a significant national debt that has be paid off (this includes money wasted on airports without airplanes, high-speed trains [AVE] without passengers, and inflated specialized tertiary care and higher educational services). Furthermore, the 2015-2018 Stability Programme update agreed between Spain and the European Union committed Spain to reducing the public health expenditure of 6% of the gross domestic product (GDP) in 2013 to 5.3% by 2018. It is true that growth can be expected in GDP, and that there could even be room for renegotiation with the EU regarding the schedule, but at present the only components of health spending expected to have a similar growth to GDP are innovative pharmaceutical products, defined as those that are nongeneric drugs, according to the agreement signed between Spain and the pharmaceutical industry in November 2015.
There is no contradiction between consolidating a human achievement, such as the welfare state, and having a dynamic leading economy. For decades, the possibility of doing so has been demonstrated by, Sweden, Norway, Finland, Denmark, and Iceland. Our welfare systems should also remove the burdens that plague them (eg, rather than subsidizing unemployment, focus should be on promoting active policies of employment and occupation, progressive taxes, etc) and follow the lead of Nordic countries.4 For example, when choosing between “for everyone” and “everything”, the European preference for universality requires a clear definition of the portfolio of services that are publicly funded. In fact, a portfolio that reflects scientific cost-effectiveness criteria and social preferences is the real factor that maintains the sustainability of the health component of the welfare state. In order to avoid dividing society into those who can pay for any innovation, regardless of its usefulness, and the vast majority who would be excluded, the introduction, maintenance, and withdrawal of health technologies needs to be based on cost-effectiveness criteria.
Firstly, cost-effectiveness analysis (CEA) provides the basis for pricing policies that encourage socially interesting innovation. These policies also create satisfaction among the citizens with the publicly funded health services who then support them via their vote. Secondly, CEA ensures the financial sustainability of the health component of the welfare state due to its desirability.
The response to the use of CEA has been wholly inadequate so far. Although CEA was formally included in the Spanish Medicines Act of 1990, it has not yet been applied in practice. Thus, in a nonbinding statement, the Spanish National Commission of Markets and Competition of November 2015,5 issued a devastating report on the draft Royal Decree on regulating the funding and pricing of medicines and medical devices and their inclusion in the pharmaceutical provision of the publicly-funded health system. In short, the report seemed to suggest that the sustainability factor of the health component of the welfare state had been abandoned. In more detail, the report drew attention to excessive administrative discretionality, the continuing lack of transparency, and the nondevelopment of the principle of cost-effectiveness, unlike the situation in the reference European countries. The principle of cost effectiveness, together with the budgetary impact, is the guarantee that the cost of pharmaceutical provision would be offset by the benefits accruing to the health of citizens. It must be remembered that the reports of regulatory bodies, such as the Spanish National Commission on Competition and Markets, assist in the development of state regulations that avoid the defects of capitalism (monopolies, cronyism) and promote its virtues (competition and innovation).
THE CORE OF THE ISSUEWhat is CEA? In relation to medical technologies, CEA is the analysis of their costs and outcomes. Three types of assessment are used to operationalize and measure outcomes. Their expression in monetary terms (eg, cost of a disease prevented by vaccination) defines a cost-benefit analysis (which indicates if it is worth more than it costs in monetary terms or not). Their expression in units of effectiveness (increased survival) defines the CEA. If the measure of effectiveness is set (ie, an additional adjusted life-year) according to a quality-of-life scale (ranging from 0 to 1, where 0 = death, and 1 = perfect health; there is a huge difference between living for 1 more year in a very disabled state and being able to perform daily activities in an acceptable state), then the analysis becomes a cost-utility analysis (CUA).6 It has become a bad habit to refer to this as a CEA.
WHY A CEA?According to a recently published analysis, all of the new drugs approved since the mid-1980s (including chemically synthesized compounds, biologics, and biosimilar drugs), represent a small fraction of those with high therapeutic added value, that is, those with marked relative efficacy and safety (ie, increased efficacy and safety compared to the drugs available).7
Regulatory agencies in most countries apply 3 criteria (barriers) to approve a medicine: efficacy, safety, and quality. However, in some countries some of the approved drugs are not funded. Budgets are limited, not all drugs can be funded, and every funding decision has an opportunity cost. The provision of health care has to be maximized using the resources available. Some countries use the cost-effectiveness ratio (the fourth barrier) as a decision rule when deciding whether to fund a new health technology. With certain qualifications, medicines whose cost-effectiveness ratio is equal to or less than a certain amount (the cost-effectiveness threshold) are covered and those with a greater cost-effectiveness ratio are excluded. According to its estimated value, this threshold is an expression of how much a given society is willing to pay for or can pay for 1 QALY. In the UK, this threshold ranges from £20 000 to £30 000 (about €30 000-€40 000) per QALY gained.8 Note that, unlike the CEA, which expresses the effectiveness of different health technologies in different ways, the CUA uses a single common denominator as a measure of outcomes (QALYs) to compare different health technologies.
To address these constraints and the growing discrepancy between the price of new drugs, which is very high in some cases, and their low incremental therapeutic value, funding agencies in a growing number of countries have begun to gradually adopt more measures: prioritizing the coverage of approved health technologies with more therapeutic value and setting their prices according to this value (ie, value-based pricing).
Firstly, this change is a signal to industry to channel their innovative capacity towards developing health technologies with higher added value and also forces the “good” regulators to “redirect” their incentives to industry towards focusing on incremental innovation. Secondly, given that regulators and funding agencies take decisions independently of each other on the approval and funding of health technologies, respectively, an increasing number of countries are managing to balance the discrepancies between approval criteria (the European Medicines Agency, the Food and Drug Administration, and agencies in Canada, Japan, Singapore, Australia, and New Zealand, among others), and pricing (how much is paid) and coverage (for whom) criteria.9
Moreover, in some countries, regulators and funding agencies have recently required manufacturers to add a budget impact analysis to the fourth barrier. This analysis consists in estimating the impact on public health spending of the adoption and diffusion of a new health technology in a given health system, taking into account its budgetary constraints and the set of provisions included in its portfolio of services within a given period. There is a good example of this in Spain.10
In summary, current regulations are changing and will undergo further change. From the perspective of society (ie, all of us), if we want to maximize our health using the resources available, we have to be efficient, and thus the incorporation and withdrawal of health technologies in and from the portfolio of services has to take into account their incremental value and price, and reflect the magnitude of this incremental value and our willingness to pay (ie, our preferences).
HOW TO PERFORM A CEAThe QALY is a measure of health that developed countries use in making funding decisions. The decision rule is to publicly fund health technologies that produce the greatest health gain (more QALYs) at a given cost.
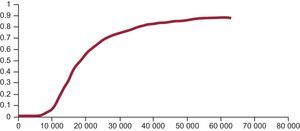
These decisions are fundamentally based on the following information. Firstly, the incremental cost-effectiveness ratio (ICER). This ratio is obtained by dividing the difference in cost between a new drug and its alternative by the difference between their QALYs (cost per QALY gained). However, like any other estimate (mean, percentage), uncertainty is always associated with estimates of costs, effectiveness, or utility. Given that the ICER is a ratio, it is complicated to calculate its confidence intervals such that this uncertainty can be expressed. Instead, other statistical methods are used that produce different possible values (estimated) of the ICER. Secondly, the acceptability curve. Given the estimated values of the ICER (the threshold), the acceptability curve is a graphic representation that indicates the probability (20%, 40%, 70%, 90%, etc) of a new health technology being cost effective (less than or equal to the set threshold) compared with the alternative for each threshold value (€20 000, €30 000, €40 000, and so on, per QALY gained)11 (Figure).
However, taking into account the instability of social preferences due to the impact of emotions and their changeability over time, the relative ignorance about how these preferences arise and how they change depending on the context in which the problem is formulated, and our strongly limited rationality, it would be wise not to rely completely on the techniques, but strengthen the procedures that allow us to establish priorities in a more democratic manner. The correct measurement of social values and preferences can also be expressed in terms of the responsible participation of citizens; that is, all of us, and not just the beneficiary segment, when establishing priorities for the allocation of publicly funded resources.12
Not long ago, some scientific societies, such as the American College of Cardiology and the European Society of Medical Oncology, began to incorporate CEA in their recommendations on the evaluation of new drugs, in addition to clinical risk-benefit analysis. The Spanish Society of Cardiology is no stranger to this trend (being a pioneer in Spain), as shown by the aims of InnovaSEC.13
The information provided by the ICER and the budget impact analysis complement each other. Because the number of patients who will benefit from a new health technology matters, if few need to be treated, then the budgetary impact of a new health technology with a very high ICER (high cost/low effectiveness, such as lapatinib) will be low. If its ICER is low, and many patients have to be treated, its budgetary impact will be high. It is worth recalling that, after the introduction of a new health technology the number of patients treated will increase, and therefore there will be an increase in the amount of information that is stored on its effectiveness and safety. So, over time, and with the emergence of new therapeutic alternatives and substitution effects, prices will change. Consequently, the ICER has to be interpreted from a dynamic perspective, as when it is compared between countries (costs vary between them).
THE CEA AND BIOLOGICS IN CARDIOLOGY: STORMY SEAS AHEAD?At an international scale, the last 2 years have seen many problems and controversies regarding new hepatitis C drugs: high estimated efficacy (for now) in the short to medium term, but high costs; more patients needing treatment; marked differences in prices between countries; a very high budgetary impact; and marked variability in how regulators and funding agencies across countries have decided which patients are covered and how to fund the treatments. Such variability leads to inefficiency, which is greater in some countries than in others. The CEA is a tool, although not the only one, that is immensely useful in improving the information available used to make difficult decisions, and therefore in helping to reduce the damage to social efficiency caused by making decisions based on insufficient or inadequate information.
It would be desirable if the same situation does not recur when addressing the stormy issues that may arise in relation to proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (a striking decrease in low-density lipoprotein levels in its indications, but high costs and specific indications that may be unfulfilled). Alirocumab and evolocumab have already been approved, bococizumab is in phase III trials, and a new chemically synthesized PCSK9 inhibiter and a PCSK9 vaccine are expected to enter clinical trials this year.14 If we follow the way in which other developed countries conduct regulation and funding, and if we apply the principles and tools described in this article, we could reduce the high risk of incurring inefficiencies that ultimately we all pay for.
SOME CONCLUSIONSIt has been well said that true innovation, accessibility, and the sustainability of health systems, to which we add solvency, are the gears of the same mechanism.15 Health systems with extensive public coverage should consolidate and maintain good long-term regulating mechanisms (R & D, approval, pricing, coverage, postmarketing surveillance and information, deinvestment, and reinvestment). In Spain, we have the technical capacity to do this. We could create an independent agency to make good decisions with legally binding power (such as the British National Institute for Health and Care Excellence). However, we do not apply all known rules of the game (added therapeutic value, ICER, budgetary impact, reinvestment), we are still not providing full accountability, we lack of transparency regarding decision-making, and we have not managed to involve all the stakeholders in these decisions.
Regulations are currently under close scrutiny, there have been many premarketing and postmarketing regulatory changes, and new proposals are underway in some countries. We must convert binary regulation (approved/not approved, funded/not funded) into a continuum of phases of obtaining information on efficacy, effectiveness, and safety (before and after conditional approval), appropriately adapt required standards of evidence to the needs of innovation and, depending on the gradually accumulated evidence, promote early access to health technologies with demonstrated efficacy and safety, change indications and the criteria governing public coverage, promptly recall health technologies when applicable, and adjust prices according to their known value at each step.9
The assessment of regulation issues and their impact on public health in Spain is more than acceptable. Sufficient structural improvement measures have been proposed, which require various kinds of resources, as well as the collaboration between all the stakeholders and strong ongoing political support.
The assessment can be of much public good: the outcomes can be used by everybody and their use by some does not prevent their use by others. Spain could even allow a temporary moratorium on the incorporation of new health technologies, like wealthier countries but, above all, like those with a more consolidated welfare state.
CONFLICT OF INTERESTSNone declared.