The Mediterranean diet, rich in polyphenols, has shown to be cardioprotective. However the mechanisms involved remain unknown. We investigated whether supplementation with a pomegranate extract rich in polyphenols renders beneficial effects on coronary function in a clinically relevant experimental model and characterized the underlying mechanisms.
MethodsPigs were fed a 10-day normocholesterolemic or hypercholesterolemic diet. Half of the animals were given a supplement of 625 mg/day of a pomegranate extract (Pomanox®; 200 mg punicalagins/day). Coronary responses to escalating doses of vasoactive drugs (acetylcholine, calcium ionophore, and sodium nitroprusside) and L-NG-monomethylarginine (endothelial nitric oxide-synthase inhibitor) were measured using flow Doppler. Akt/endothelial nitric oxide-synthase axis activation, monocyte chemoattractant protein-1 expression, oxidative deoxyribonucleic acid damage in the coronary artery, and lipoprotein resistance to oxidation were evaluated.
ResultsIn dyslipidemic animals, Pomanox® supplementation prevented diet-induced impairment of endothelial relaxation, reaching vasodilatory values comparable to normocholesterolemic animals upon stimulation with acetylcholine and/or calcium ionophore. These beneficial effects were associated with vascular Akt/endothelial nitric oxide-synthase activation and lower monocyte chemoattractant protein-1 expression. Pomanox® supplementation reduced systemic oxidative stress (higher high-density lipoprotein-antioxidant capacity and higher low-density lipoprotein resistance to oxidation) and coronary deoxyribonucleic acid damage. Normocholesterolemic animals elicited similar drug-related vasodilation regardless of Pomanox® supplementation. All animals displayed a similar vasodilatory response to sodium nitroprusside and L-NG-monomethylarginine blunted all vasorelaxation responses except for sodium nitroprusside.
ConclusionsPomanox® supplementation hinders hyperlipemia-induced coronary endothelial dysfunction by activating the Akt/endothelial nitric oxide-synthase pathway and favorably counteracting vascular inflammation and oxidative damage.
Keywords
Cardiovascular disease (CVD) is the leading cause of mortality worldwide and atherosclerosis stands as one of its major underlying causes.1 The endothelium plays a fundamental role in atherosclerosis prevention by regulating the vascular tone, leukocyte adhesion, and thrombus formation. In fact, endothelial dysfunction, believed to be a consequence of repeated exposure to cardiovascular risk factors (particularly hypercholesterolemia), is considered the hallmark of early atherosclerosis and is present even prior to the appearance of vascular lesions.2,3 Furthermore, endothelial dysfunction has been shown to be a predictor of adverse outcome in patients with coronary artery disease.4 Hence, strategies aimed at preventing or reducing endothelial damage have become a focus of attention.
Several epidemiological studies have evidenced that adherence to a healthy dietary pattern characterized by relatively high intake of fruits and vegetables is associated with a reduction in the incidence of CVD.5,6 In fact, the existing data indicates that the role of fruits and their associated nutrients in cardiovascular prevention could be stronger than that of vegetables. In contrast, neutral and negative results have been obtained in controlled clinical trials failing to demonstrate significant CVD prevention with vitamin and antioxidant supplementations, underscoring the importance of whole foods.7 Experimental and mechanistic evidence suggests that fruits present an array of disease-preventive phytochemicals, such as polyphenols, which contribute to the apparent modulation of atherosclerotic risk factors and atherosclerosis development.8–10 In this regard, within the last decade, pomegranate (Punica granatum L.) has gained widespread popularity as a polyphenol-rich food with health-promoting properties.11 Most of the pomegranate health benefits have been attributed to the presence of ellagitannins (mainly the large polyphenol compounds punicalagins isomers α and β), which are unique to pomegranate.12 Although ellagitannins are not absorbed, under physiological conditions they become hydrolyzed to ellagic acid, which in turn is gradually metabolized by the intestinal microbiota to produce different types of urolithins (metabolites). Urolithins are thought to be responsible for the benefits associated with pomegranate consumption.13,14
In the present study we sought to investigate the in vivo effects of a pomegranate extract rich in punicalagins (namely Pomanox® [POX]) on vascular protection and to elucidate its underlying mechanisms. Indeed, whether pomegranate exerts vascular beneficial effects has yet to be determined. We carried out our study in a porcine model of coronary vasoreactivity fed either a regular chow or a high fat/high cholesterol-diet. Research using relevant animal models with translational clinical impact is needed to better determine and further explore the biological mechanisms through which polyphenol-rich foods may exert their clinical effects.
METHODSThe study protocol was approved by the institutional ethics committee (Consejo Superior de Investigaciones Científicas-Institut Català de Ciències Cardiovasculars) and all procedures fulfilled the criteria established by the “Guide for the care and use of laboratory animals” (National Institute of Health publication number 85-23, revised in 1996).
Study DesignCrossbred commercial female swine (48 [3] kg) were fed during 10 days a standard pig chow (normocholesterolemic [NC] diet, N = 12) or a high fat/high cholesterol diet (Western-type hypercholesterolemic [HC] diet, N = 12) of 20% saturated fat, 2% cholesterol, 1% cholic acid). We have already reported that intake of this fat-rich diet for 10 days raises cholesterol to levels comparable to that found in dyslipidemic humans and induces endothelial dysfunction.15 Half of the NC and HC animals were provided a supplement of 625mg/day POX. Four experimental groups (6 animals per group) were studied: NC; NC + POX; HC; and HC + POX. All animals were carefully monitored (ie, continuous supervision) to ensure the daily consumption of POX throughout the study. Pomanox® is a pomegranate extract standardized by its punicalagins α + β content. Pigs were supplemented during 10 days with a POX extract (punicalagins content of 32.21%), which corresponds to 200mg punicalagins/day. This dose was chosen based on previous studies in humans, which used doses ranging from 78mg/day punicalagins (tested in coronary artery disease patients supplemented during 3 years and diabetic patients during 3 months)16 to 380mg/day (healthy patients during 4 weeks).17 The POX was provided by Probelte Biotecnologia S.L. (Spain).
On day 10, at 1 h post-dietary ingestion, coronary endothelium-dependent and –independent vasodilation was evaluated in vivo by catheter-based infusion of vasoactive substances into the left anterior descending coronary artery as previously described.15 As to the treatment schedule, Seeram et al18 reported that maximal plasma concentrations of a punicalagin-related metabolite, ellagic acid, were reached at 1 h after consumption of pomegranate fruit juice. At the end of the experimental procedure, animals were euthanized with an overdose of potassium chloride.
Preparation and Characterization of Pomanox®POX was prepared from freshly harvested pomegranate fruits growing in the Spanish Mediterranean region according to the European Patent EP1967079.19 Punicalagins α + β quantification was performed by high-performance liquid chromatography/reverse phase chromatography with diode array detection. The POX specifications are shown in Table 1A of the supplementary material. The analytical method was validated by Probelte Biotecnología S.L., in terms of linearity, repeatability, reproducibility, recovery and specificity, according to regulatory guidelines: ICH Topic Q 2 R1 “Validation of analytical procedures: text and methodology”, (November 6, 1996, incorporated in November 2005; Table 1B of the supplementary material).
Vascular Reactivity StudiesTen days after the diet regime and 1 h after the last diet intake (with or without POX supplementation), pigs were sedated with an intramuscular injection of tiletamine + zolazepam (7mg/kg) + medetomidine (0.07mg/kg), endotracheally intubated, and anesthesia was maintained with isofluorane (2%). Under aseptic conditions, an incision was made in the ventral portion of the neck to expose the carotid artery and another incision was performed in the thorax to proceed with the opening of the chest, pericardium removal, and heart exposure. Thereafter, an ultrasonic flow probe connected to a blood flow meter (Two-Channel Perivascular Flow System; ADInstruments) was carefully placed in the mid-portion of the dissected left anterior descending coronary artery. A second flow probe was placed in the carotid artery in order to simultaneously measure flow changes in larger vessels for control purposes (peripheral assessment). After vessel diameter and complete hemodynamic baseline measurements were performed, vascular reactivity was assessed by intracoronary delivery of vasoactive agents. To this end, animals underwent catheterization of the left main coronary artery. All drugs were diluted with physiologic 0.9% NaCl solution to a volume of 1ml and were infused during a 30-second period. There was at least a 15-minute interval between completion of one drug infusion and administration of the next. Endothelial-dependent vasodilatation was assessed by the intracoronary infusion of acetylcholine (receptor-operated vasodilator; 10-9 mmol/L to 10-6 mmol/L, Sigma) and calcium ionophore A23189 (nonreceptor-operated vasodilator; 10-9 mmol/L to 10–6 mmol/L, Sigma), whereas the endothelium-independent vasodilatation (vascular smooth muscle-related) was assessed with a dose-response curve to SNP (sodium nitroprusside, 10-7 mmol/L to 10-5 mmol/L). The doses of vasoactive substances, while producing the desired effects following intracoronary administration as previously reported,15,20 did not induce any changes at a systemic level. The role of nitric oxide synthase pathway in the relaxation responses was assessed through the addition of L-NMMA (L-NG-monomethylarginine); 10mg/kg; Sigma), an nitric oxide-synthase (NOS) inhibitor. Data are presented as the percent change of coronary blood flow response measurements from baseline to maximal postpharmacological agent infusion (i.e., percentage of relaxation). Femoral mean blood pressure and heart rate were continuously monitored by a blood pressure transducer and an electrocardiogram throughout all the procedure.
Molecular Analysis of Endothelial MarkersAt sacrifice, the left anterior descending coronary artery of all animals was carefully isolated. One portion was snap frozen in liquid nitrogen for molecular analysis of endothelial-related markers and the other portion was fixed in 4% paraformaldehyde and paraffin-embedded for immunohistochemical analysis of DNA oxidative damage.
Real Time Polymerasse Chain ReactionGene levels of endothelial NOS (eNOS) and monocyte chemoattractant protein-1 (MCP-1) were assessed in the coronary artery of all animals. Gene expression was evaluated by the ABIPRISM real-time PCR-7000 Sequence Detection System (Applied Biosystems). The threshold cycle values were determined and normalized to the housekeeping gene 18SrRNA.
Western Blot AnalysisWe assessed protein expression of Akt/PKB (Santa Cruz, #C-20) and Akt phosphorylated at Ser473 (Cell Signaling, #9271) as well as its downstream effector eNOS (Cell Signaling, 9572#) and its active form, eNOS phosphorylated at Ser1177(Cell Signaling, #9571). The MCP-1 protein content was also assessed (R&D System, #P0161) and corrected for β-actin. Densitometric analyses were performed with the software ImageJ.
Coronary Oxidative DamageA 8-hydroxyguanosine (Abcam ab48508) staining was performed in paraffin-embedded coronary samples. Images were captured by Nikon Eclipse 80i microscope and digitized by Retiga 1300i Fast camera.
Systemic Oxidative MarkersLow-density Lipoproteins OxidationThe resistance of low-density lipoproteins (LDL) against copper-induced oxidation was determined in EDTA (ethylenediaminetetraacetic acid)-blood samples collected from all animals at baseline and at the end of the 10-day diet period.21 We also determined the lipid peroxide content of oxidized-LDL by assessing thiobarbituric-acid-reactive substances.
High-density Lipoproteins Antioxidant ActivityHigh-density lipoproteins (HDL) antioxidant capacity was assessed in HDL isolated from serum of all animals at baseline and on day 10 by assessing HDL total radical-trapping antioxidative potential as previously described.22,23 This method is based on the capability of HDL to protect LDL against oxidation (control LDL).
Statistical AnalysisResults are reported as mean (standard error of the mean). After testing for normal distribution of the data with the Shapiro-Wilk normality test, statistical significance was determined through a one-way analysis of variance, followed by Fisher protected least-significant difference post-hoc analysis or with Student t test for paired data as required. Values of P < .05 were considered statistically significant. All statistical analysis was performed with the Statview software package.
RESULTSClinical, Biochemical, and Hematological DeterminationsWeight gain throughout the study was comparable among the four animal groups (NC, 4.3 [0.9] kg; NC + POX, 3.8 [0.4] kg; HC, 3.9 [0.5] kg; HC + POX, 4.1 [0.4] kg). As shown in Table A the short-term (10 days) administration of this high-cholesterol/high-fat diet significantly raised mean plasma cholesterol levels, LDL-cholesterol, and HDL-cholesterol as compared to baseline values. This western-type hypercholesterolemic regime led to a total-cholesterol/HDL-cholesterol ratio ≈ 4 and non-HDL of > 280mg/dL, values similar to those found in humans with hypercholesterolemia. Glucose and triglyceride levels remained unaltered across all groups of animals throughout the study. No changes were detected in kidney- and liver-related parameters (Table B) nor in hematological counts throughout the study (Table 2 of the supplementary material). Previous longer-term follow-up studies have already supported the safety of punicalagins administration.24
Follow-up of Glucose and Lipid Levels and Liver and Kidney Parameters
| A. Glucose and lipids | Glucose (mg/dL) | Triglycerides (mg/dL) | Cholesterol (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | LDL/HDL | Non-HDL cholesterol (mg/dL) | Total cholesterol/HDL ratio |
|---|---|---|---|---|---|---|---|---|
| HC | ||||||||
| Control | ||||||||
| Baseline | 112 (19) | 24 (4) | 101 (3) | 66 (7) | 52 (10) | 0.8 (0.1) | 34.7 (5) | 1.6 (0.2) |
| Experimental day | 111 (10) | 26 (2) | 395 (20)a | 94 (7)a | 286 (140)a | 2.8 (1.1)a | 301.0 (25)a | 4.2 (0.3)a |
| POXb | ||||||||
| Baseline | 137 (25) | 21 (4) | 114 (5) | 54 (3) | 50 (7) | 0.9 (0.1) | 48.0 (7) | 2.0 (0.1) |
| Experimental day | 135 (11) | 19 (4) | 475 (41)a | 113 (9)a | 353 (114)a | 3.2 (1.1)a | 361.0 (54)a | 4.2 (0.2)a |
| NC | ||||||||
| Control | ||||||||
| Baseline | 84 (13) | 27 (8) | 96 (10) | 36 (3) | 64 (4) | 1.1 (0.2) | 64.0 (10) | 2.4 (0.3) |
| Experimental day | 76 (11) | 24 (15) | 95 (8) | 32 (3) | 32 (5) | 1.1 (0.2) | 63.0 (4) | 2.6 (0.3) |
| POXb | ||||||||
| Baseline | 111 (10) | 39 (5) | 109 (7) | 47 (5) | 47 (4) | 1.0 (0.2) | 54.0 (3) | 2.4 (0.4) |
| Experimental day | 115 (24) | 35 (4) | 102 (4) | 48 (3) | 41 (4) | 1.3 (0.6) | 53.0 (11) | 2.7 (0.7) |
| HC, increase vs baseline | ||||||||
| Control | 27 (7)c | 341 (97)d | ||||||
| POXb | 59 (9)c | 303 (133)d | ||||||
| B. Liver and kidney parameters | Urea (mg/dL) | Creatinine (mg/dL) | GGT (U/L) | GOT (U/L) | GPT (U/L) | |||
|---|---|---|---|---|---|---|---|---|
| HC | ||||||||
| Control | ||||||||
| Baseline | 14 (1) | 1.7 (0.1) | 38 (7) | 19 (3) | 38 (2) | |||
| Experimental day | 34 (5) | 1.8 (0.1) | 36 (5) | 37 (12) | 36 (5) | |||
| POXb | ||||||||
| Baseline | 14 (3) | 1.2 (0.2) | 36 (5) | 26 (6) | 35 (4) | |||
| Experimental day | 27 (3) | 1.3 (0.1) | 32 (3) | 28 (6) | 36 (2) | |||
| NC | ||||||||
| Control | ||||||||
| Baseline | 18 (3) | 1.1 (0.1) | 37 (3) | 25 (4) | 34 (4) | |||
| Experimental day | 20 (4) | 1.3 (0.1) | 40 (5) | 24 (3) | 29 (4) | |||
| POXb | ||||||||
| Baseline | 17 (1) | 1.3 (0.1) | 30 (1) | 24 (5) | 24 (2) | |||
| Experimental day | 17 (2) | 1.2 (0.1) | 28 (4) | 23 (4) | 26 (4) | |||
GGT, gamma-glutamyl transferase; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; HC, hypercholesterolemic; HDL, high-density lipoproteins; LDL, low-density lipoproteins; NC, normocholesterolemic;
POX, Pomanox®.
Data are expressed as mean (standard error of the mean).
All animals presented a similar size of coronary (median value: 0.25 [0.06] cm) and carotid (median value: 0.32 [0.01] cm) arteries.
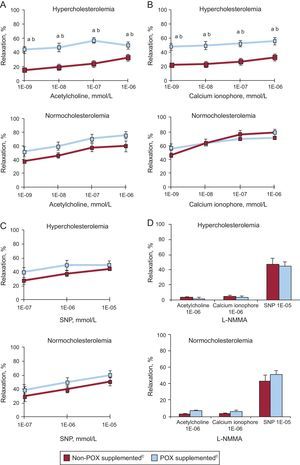
Animals fed a 10-day HC diet developed coronary endothelial dysfunction, as evidenced by a marked impairment (50% reduction) in acetylcholine- (Figure 1A) and calcium- (Figure 1B) induced relaxation capacity as compared to NC animals (P < .05). This vasodilatory impairment was evident at all tested doses of both vasoactive agents in a dose-dependent manner (Figures 1A and 1B).
Graphs showing relaxation response to (A) acetylcholine, (B) calcium ionophore, (C) sodium nitroprusside, and (D) L-NG-monomethylarginine in the coronary artery of normolipemic and hypercholesterolemic animals. L-NMMA, L-NG-monomethylarginine; POX, Pomanox®; SNP, sodium nitroprusside. Data are expressed as a percent relaxation from baseline measurement (mean [standard error of the mean]).
aP < .05 vs normocholesterolemic-fed animals.
bP < .05 vs hypercholesterolemic-control fed animals.
cN = 6 animals per group.
The POX supplementation significantly restored endothelial-dependent vasodilatory capacity in those animals fed a HC diet, reaching percentages of coronary relaxation similar to those encountered in NC animals (P < .05 vs HC; P = .87 vs NC). Indeed, POX supplementation improved both receptor-mediated (acetylcholine-induced) and non-receptor operated (calcium ionophore-induced) vasodilation in all dyslipidemic animals.
In contrast, all healthy NC animals displayed comparable vasodilatory responses regardless of POX supplementation and no further acetylcholine- and calcium ionophore- mediated vasodilatory effect was observed in POX-fed animals (Figures 1A and 1B).
All four groups of animals exhibited a similar dose-dependent relaxation in response to SNP (Figure 1C), supporting no influence on vascular smooth muscle function as well as POX-related effects centered on endothelial cell function.
Pretreatment with L-NMMA nearly abolished the vasodilatory effect elicited by high doses of acetylcholine and calcium ionophore in all animals, whereas the addition of exogenous NO (nitric oxide) (ie, SNP) restored the coronary relaxation response (Figure 1D), further indicating an endothelial NO-driven effect.
Carotid flow remained unaltered throughout the whole experimental period (2% [1]% variation with respect to baseline measurements). Similarly, femoral pressure and heart rate were unaffected after intracoronary administration of the vasoactive agents in all animals (Table 3 of the supplementary material).
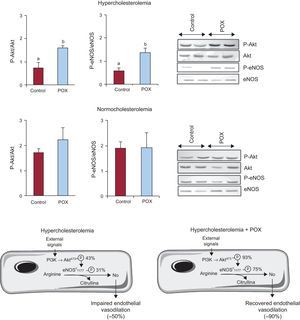
Pomanox® mechanisms of action on the coronary arteryAkt/Endothelial Nitric Oxide-synthase AxisDyslipidemia lead to a significant reduction of the Akt/eNOS axis activation as compared to NC animals (Figure 2; P < .05), yet, this function was almost completely restored by POX supplementation. NC + POX animals showed a similar degree of Akt/eNOS activation to that detected in NC-control animals (Figure 2). No changes were observed in eNOS mRNA levels in all animal groups (HC, 1.7 [0.6]; HC + POX, 1.7 [0.5]; NC, 1.6 [0.4]; NC + POX, 1.2 [0.3] eNOS, mRNA/18SrRNA).
Activation of the Akt/endothelial nitric oxide-synthase axis in the coronary artery of hypercholesterolemic and normocholesterolemic-fed animals and representative western blot image. Diagram illustrating the mechanisms involved in endothelial nitric oxide-synthase activation and subsequent nitric oxide release and the levels of expression of the Akt/endothelial nitric oxide-synthase axis in hypercholesterolemic-fed animals (with and without Pomanox®), taking as 100% healthy regular-fed animals (normocholesterolemic controls; N = 6 animals per group). eNOS, endothelial nitric oxide-synthase; POX, Pomanox®.
aP < .05 vs normocholesterolemic-fed animals.
bP <.05 vs hypercholesterolemic-control fed animals.
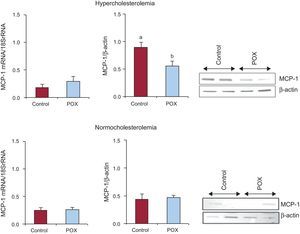
The HC animals showed an increased coronary MCP-1 protein content as compared to NC (P < .05; Figure 3). However, POX supplementation significantly decreased, by about 50%, the expression of this inflammatory chemokine in dyslipidemic animals to levels comparable to those found under non-pro-atherogenic conditions (Figure 3). No changes in gene-expression levels were observed among all animals.
Effect of Pomanox® supplementation in monocyte-chemoattractant protein-1 coronary content (mRNA and protein expression) in hypercholesterolemic - and normocholesterolemic - fed animals (N = 6 animals per group). MCP-1, monocyte-chemoattractant protein-1; POX, Pomanox®.
aP < .05 vs normocholesterolemic-fed animals.
bP < .05 vs hypercholesterolemic-control fed animals.
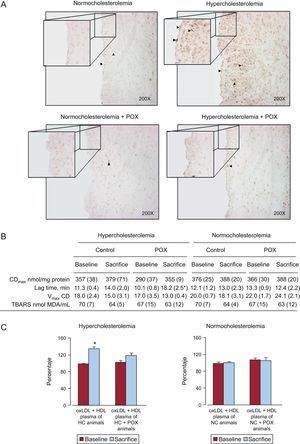
As shown in Figure 4A, 8-OHdG positive cells were significantly increased in the coronary arteries of all HC animals as compared to NC, affecting both the endothelial layer and the intima. The POX supplementation in hyperlipemic animals protected against the oxidative damage induced by hypercholesterolemia because the number of 8-OHdG positive cells was negligible, as observed in healthy animals fed a NC diet.
Pomanox® antioxidative potential. A: coronary DNA oxidative damage assessment by 8-OH-dG. Pomanox® supplementation prevented hyperlipemia-induced vascular oxidative stress in both endothelial cells and intima (arrowheads). These representative images perfectly reflect what was constantly observed within the different animal groups. B: oxidation of low density lipoprotein particles. C: high density lipoproteins-antioxidant potential expressed as percentage of low density lipoprotein oxidation (control oxidized low density lipoproteins ≈ 100%) (N = 6 animals per grpup). CDmax, maximal conjugated dienes; HDL, high density lipoproteins; LDL, low density lipoproteins; oxLDL, oxidized low density lipoproteins; POX, Pomanox®; TBARS, thiobarbituric-acid-reactive substances; Vmax CD, maximal velocity of conjugated diene formation; *P < .05 vs baseline.
The POX supplementation lead to a significant (P < .05) increase in LDL resistance to in vitro oxidation, determined as a prolongation of the lag time (almost 2 times longer) from baseline to the end of the 10-day POX administration period, in those animals fed HC diet (Figure 4B). The LDL oxidation was not affected by POX supplementation under NC conditions.
No differences were detected in the capacity of LDL to reach oxidation (maximal conjugated dienes and maximal velocity of conjugated diene formation) or in thiobarbituric-acid-reactive substances among the four animal groups (Figure 4B).
The HDL isolated from HC-fed animals supplemented with POX showed significantly higher antioxidant activity against LDL oxidation as compared to non-POX supplemented HC animals, reaching levels comparable to those observed at baseline (Figure 4C). In contrast, The HDL particles isolated from HC-fed animals could not counteract LDL oxidation, displaying a significantly lower antioxidant potential as compared to baseline. No changes were observed in NC-fed animals (with and without POX supplementation).
DISCUSSIONIn the present study we demonstrate, in an in vivo porcine model with human resemblance, that diet supplementation with a pomegranate extract rich in punicalagins (POX, 200mg punicalagins/day) prevents hyperlipemia-induced impairment of endothelium-dependent coronary vasorelaxation, a beneficial effect involving activation of the Akt/eNOS axis, lower MCP-1 expression, and decreased oxidative damage in the coronary arteries as well as an overall decline in systemic oxidative stress. Conversely, POX supplementation to healthy animals fed regular chow with fully functional endothelial cells does not elicit any effects.
Here we demonstrate in a preclinical animal model by coronary flow-reactivity assessment that supplementation with POX during a short period of time (10 days) prevents hypercholesterolemia-induced coronary endothelial dysfunction, acquiring vascular vasodilatory capacity equal to that observed in normal, healthy, regular-fed animals. Indeed, no further advantages in vasorelaxation are detected in healthy animals fed a NC diet because their endothelium is already fully functional at baseline. Yet, previous human studies have shown that individuals at a higher risk of coronary heart disease seem to obtain greater benefits from pomegranate properties than healthy volunteers.25,26
There is a growing body of evidence showing that disruption of the eNOS activation pathway and/or reduced availability of NO contribute to endothelial dysfunction, the hallmark of atherosclerosis.27 We demonstrate that POX restoration of hypercholesterolemia-induced coronary endothelial dysfunction in swine is associated with the activation of Akt/eNOS axis. Indeed, POX induces NO-mediated relaxation by a mechanism involving endothelial activation of Akt and eNOS phosphorylation at Ser1177 (ie, eNOS activation) at levels comparable to those found under normal conditions. Interestingly, no changes are observed in total NOS protein levels. Moreover, eNOS activation not only is phosphorylation-dependent but is also coupled to a raise in intracellular Ca2+.28 We report that POX supplementation in dyslipemic animals restores both muscarinic receptor- and A23187 (a Ca2+ ionophore)–dependent eNOS stimulation, likely involving intracellular calcium increase.28 Because many intracellular enzymes are likely to metabolize punicalagins, their effects upon the activation of eNOS may be due to their derived metabolites. Whether punicalagin-related metabolites (eg, urolithins) can indeed induce eNOS activity requires further investigation.
Oxidative stress, an imbalance between free radical formation and antioxidant capacity, is a major contributor to CVD that also triggers inflammatory reactions.29 Oxidative stress induces inflammation by acting on the pathways that generate inflammatory mediators like adhesion molecules and pro-inflammatory cytokines/chemokines (eg, MCP-1). Recent studies in CVD patients have shown significant positive associations between oxidative stress and inflammation and indicators of vascular damage, like impaired endothelial function.30
Our findings show that short-term intake of punicalagins abrogated 8-OHdG positive cells in the endothelial layer and intima of the coronary arteries of dyslipidemic animals, indicating protection against hypercholesterolemia-induced cellular damage in the vasculature. 8-OHdG has shown to serve as a sensitive biomarker of intracellular DNA-damage induced by oxidative stress in vivo.31 Meanwhile, we also find significant increase in the resistance of circulating LDLs to oxidation and a higher HDL antioxidant potential. These local and systemic antioxidant effects elicited by POX under hyperlipemic conditions in combination with a marked decline in coronary MCP-1 expression (induced by HC diet) may help to explain the detected improvement in coronary function. The MCP-1 is known to play a critical role in the initiation of atherosclerosis by mediating monocyte recruitment to the damaged vessels.32 So far, pomegranate has shown to elicit anti-inflammatory activity by suppressing inflammatory cytokine/chemokine production in cancer cells33 and in the Caco-2 in vitro intestinal model.34
All together, our observations support that POX supplementation may retard the appearance of atherosclerotic disease. In fact, dietary polyphenols, rather than vitamins and beta-carotenes, seem to be more effective in cardioprotection.7 Studies in patients with carotid artery stenosis who consumed pomegranate juice during 3 years clearly demonstrated a reduction in atherosclerotic lesion size in addition to reduced serum oxidative stress and increased serum paraoxonase activity (HDL-associated antioxidant enzyme).35
As for the potential effect of pomegranate in the treatment of hyperlipemia/glycemia, we report that a 10-day oral supplementation with POX does not affect glucose levels but renders a better lipid profile trend (Table). Daily administration of pomegranate juice (equal to 1.5 mmol total polyphenols) during 3 months in patients with type 2 diabetes mellitus led to no variations in lipid profile and glucose levels.16 However, in contrast, consumption of 40g of pomegranate daily during 2 months by patients with type 2 diabetes mellitus and manifest hyperlipemia resulted in a significant decrease in total and LDL-cholesterol, although no changes were reported in HDL-cholesterol or glucose levels.36 These differing observations might be explained by pomegranate composition or dosages used as well as duration of administration. The punicalagin content of pomegranate juice depends on many factors (the variety of pomegranate, the harvest time, juice processing, etc)37 and around 3-4 fruits (340ml pomegranate squeezed juice) would be required to fulfill the herein evaluated doses of punicalagins (200mg). Longer POX-supplementation periods may also provide additional clear benefits in lipid parameters.
Finally, the potential health benefit of pomegranate, whether as a whole fruit, juices, or whole fruit extracts, is supported by several small-scale human studies showing potential beneficial effects on CVD, cancer, diabetes, dental conditions, bacterial infections, and antibiotic resistance, among others.38 Our findings may partly explain the biological mechanisms behind the promising cardiovascular health effects detected after daily and chronic pomegranate supplementation in humans. As such, improvement in coronary Akt/eNOS signaling and further NO release in concurrence with a reduction in oxidative stress and inflammation may have contributed to the improvement in stress-induced myocardial ischemia detected in patients with coronary heart disease25 as well as the reduced atherosclerotic burden observed in patients with carotid artery stenosis.35
CONCLUSIONSAltogether, our study supports that inclusion of POX in the diet may retard the development of vascular dysfunction and atherosclerosis in early stages in subjects eating a fat-rich diet. Indeed, our results also show that the potential benefits of this polyphenol-enriched supplement are only detected under a hyperlipemic setting. Therefore, we may speculate that the clinical impact of POX intake in statin-treated patients with controlled lipid profile would be underestimated by statins, especially taking into account that statins have already proven to improve endothelial dysfunction either by directly modulating lipid parameters or through the well-known lipid-unrelated effects (“pleiotropic effects”).39
It is worth mentioning that available results indicate a wide presence of vascular disease among adolescents as well as in healthy adults.40 The fact that pomegranate exerts protective effects against early vascular dysfunction suggests that it may be an effective nutraceutical, both in patients with cardiovascular risk factors and in individuals who are following a heart-protection healthy lifestyle.
FUNDINGThis work was supported by the PNS (Programa Nacional de Salud)—SAF2013-42962-R project awarded to L. Badimon—and PNS-SAF2012-40208—to Gemma Vilahur, and CEN-20101016 (HENUFOOD) from CDTI-MINECO (Centro para el Desarrollo Tecnológico Industrial-Ministerio de Competitividad y Economía) (to L. Badimon). G. Vilahur is a Ramon y Cajal program researcher under contract with the MICINN (RyC-2009-5495, MICINN, Spain).
CONFLICTS OF INTERESTJ.A. López and S. Streitenberger are employees of Probelte Biotecnología S.L.
All authors have read and approved the final manuscript. The support provided by P. Catalina, M.A. Canovas, F.J. Rodriguez, J.J. Andres, O.J. Babot, and M.A Velasco with animal handling and care and for the proper conduct of the experimental and molecular work is gratefully and highly recognized.
We thank Probelte Biotecnología S.L. for providing Pomanox®.
We thank Fundación Jesus Serra, Barcelona, for their continuous support.

![Graphs showing relaxation response to (A) acetylcholine, (B) calcium ionophore, (C) sodium nitroprusside, and (D) L-NG-monomethylarginine in the coronary artery of normolipemic and hypercholesterolemic animals. L-NMMA, L-NG-monomethylarginine; POX, Pomanox®; SNP, sodium nitroprusside. Data are expressed as a percent relaxation from baseline measurement (mean [standard error of the mean]). aP < .05 vs normocholesterolemic-fed animals. bP < .05 vs hypercholesterolemic-control fed animals. cN = 6 animals per group. Graphs showing relaxation response to (A) acetylcholine, (B) calcium ionophore, (C) sodium nitroprusside, and (D) L-NG-monomethylarginine in the coronary artery of normolipemic and hypercholesterolemic animals. L-NMMA, L-NG-monomethylarginine; POX, Pomanox®; SNP, sodium nitroprusside. Data are expressed as a percent relaxation from baseline measurement (mean [standard error of the mean]). aP < .05 vs normocholesterolemic-fed animals. bP < .05 vs hypercholesterolemic-control fed animals. cN = 6 animals per group.](https://static.elsevier.es/multimedia/18855857/0000006800000003/v1_201502251116/S1885585714003284/v1_201502251116/en/main.assets/thumbnail/gr1.jpeg?xkr=eyJpdiI6Iml6Vnh2Sk5JT2JZeGJ5eG1LY1JDU0E9PSIsInZhbHVlIjoiREJsdzIvUytFcUxxUWpuaXMrdUVGbUNYTnI2cDA0ZEdVUWtHODRSSDNXWT0iLCJtYWMiOiIzZjZmOTFkYjQ3MDVlYzUzMTUxOGE2NTI0ZmQ4YjhlNTZjM2Q1YjJkYWRhMjBlNjIxMTYwMzA0NTQxZDBiNTA3IiwidGFnIjoiIn0=)


