Keywords
INTRODUCTION
Atherosclerosis is the major underlying cause of ischemic coronary artery disease and platelets play a key role in atherothrombotic complications occurring in patients with acute coronary syndromes (ACS) and in those undergoing percutaneous coronary intervention (PCI).1-3 Following atherosclerotic plaque rupture, platelet mediated thrombosis occurs through a 3-step process: adhesion, activation, and aggregation. Each of these phases represents a target for the development of antiplatelet agents. Inhibitors of platelet adhesion are still under investigation and not approved for clinical use. Inhibitors of platelet aggregation (ie, intravenous glycoprotein IIb/IIIa inhibitors) are reserved only for the acute phase treatment of high risk ACS patients undergoing PCI. Inhibitors of platelet activation processes represent the mainstay treatment for the acute and long-term prevention of recurrent ischemic events in ACS and PCI patients.
Currently, 2 groups of platelet activation inhibitors, aspirin and thienopyridines, are clinically approved for prevention of recurrent ischemic events in ACS/PCI patients. Aspirin (acetylsalicylic acid) inhibits platelet activation through irreversible blockade of the cyclooxygenase (COX)-1, which in turn prevents production of thromboxane A2. The benefit of aspirin therapy for short and long-term secondary prevention of thrombotic events has been extensively proven.4,5 However, the elevated recurrence rate of ischemic events, particularly in high risk settings, sets the basis for the development of antiplatelet drugs that target other pivotal signaling pathways such as those mediated by adenosine diphosphate (ADP). Thienopyridines represents a class of antiplatelet agents that inhibit the P2Y12 ADP receptor subtype and are now the cornerstone of treatment as an adjunct to aspirin in ACS/PCI patients. Clopidogrel is currently the thienopyridine of choice. Despite the clinical benefits observed with adjunctive clopidogrel treatment, shortcomings have also been identified with this drug.6,7 The present manuscript provides an overview on the current status and future directions in P2Y12 receptor antagonism, with particular emphasis on interindividual variability in response to clopidogrel and strategies, such as novel antiplatelet agents, to improve P2Y12 inhibition.
PLATELET PURINERGIC RECEPTORS
Purinergic receptors expressed on platelets consist of P2X1, P2Y1, and P2Y12. Adenosine triphosphate (ATP) is the physiological agonist of P2X1. a ligand-gated cation channel. P2X1 is involved in platelet shape change through extracellular calcium influx and helps to amplify platelet responses mediated by other agonists.8 ADP is the physiological agonist and, thus, exerts its action on platelets through both G protein-coupled seven transmembrane domains purinergic receptors, P2Y1 and P2Y12.9,10 Activation of the P2Y1 receptor leads to a transient change in platelet shape, intracellular calcium mobilization, granule release of other mediators and finally initiates a weak and transient phase of platelet aggregation.8,9 Although both P2Y receptors are needed for complete aggregation,11 ADP-stimulated effects on platelets are upheld predominantly by the Gi-coupled P2Y12 receptor signaling pathway. Activation of P2Y12 receptors causes a series of intracellular events that result in calcium mobilization, granules release, thromboxane A2 generation and activation of glycoprotein IIb/IIIa receptor, which leads to amplification of platelet aggregation and stabilization of the platelet aggregate.10-12 Therefore, platelet P2Y12 blockade is pivotal in order to inhibit platelet activation and aggregation, thus, preventing formation of platelet thrombus (Figure 1).
Figure 1. Purinergic receptors and mechanism of action of clopidogrel. Clopidogrel is a pro-drug of which approximately 85% is hydrolyzed by esterases in the blood to inactive metabolites and only 15% is metabolized by the cytochrome P450 (CYP) system in the liver into an active metabolite. The active metabolite irreversibly inhibits the adenosine diphosphate (ADP) P2Y12 receptor. The P2X1 receptor, which uses adenosine triphosphate (ATP) as an agonist, is involved in platelet shape change through extracellular calcium influx and helps to amplify platelet responses mediated by other agonists. Activation of the P2Y1 receptor leads to alteration in shape and initiates a weak and transient phase of platelet aggregation. The binding of ADP to the Gq-coupled P2Y1 receptor activates phospholipase C (PLC), which generates diacylglycerol (DAG) and inositol triphosphate (IP3) from phosphatidylinositol biphosphate (PIP2). Diacylglycerol activates protein kinase C (PKC) leading to phosphorylation of myosin light chain kinase (MLCK-P) and IP3 leads to mobilization of intracellular calcium. The P2Y1 receptor is coupled to another G-protein, G12, which activates the "Rho" protein and leads to the change in platelet shape. The binding of ADP to the Gi-coupledP2Y12 receptor liberates the Gi protein subunits ai and bg, resulting in stabilization of platelet aggregation. The ai subunit inhibits adenylyl cyclase (AC) and, thus, reduces cyclic adenosine monophosphate (cAMP) levels, which diminishes cAMP-mediated phosphorylation of vasodilator-stimulated phosphoprotein (VASP-P). The status of VASP-P modulates glycoprotein (GP) IIb/IIIa receptor activation. The subunit bg activates the phosphatidylinositol 3-kinase (PI3K), which leads to GP IIb/IIIa receptor activation through activation of a serine-threonine protein kinase B (PKB/Akt) and of Rap1b GTP binding proteins. Prostaglandin E1 (PGE1) activates AC, which increases cAMP levels and status of VASP-P. Solid arrows indicate activation; dotted arrows indicate inhibition. With permission from Angiolillo DJ et al.6
P2Y12 Receptor Antagonism
Thienopyridines are non-direct and irreversible P2Y12 receptor inhibitors, and represent the only P2Y12 blockers currently approved for clinical use. Ticlopidine, a first-generation thienopyridine, in combination with aspirin was proven superior to aspirin alone or anticoagulation in combination with aspirin in the setting of PCI.13-16 Due to safety concerns, mainly high rates of neutropenia, ticlopidine was soon widely replaced by clopidogrel, a second-generation thienopyridine with similar efficacy and a better safety profile.17 In addition, clopidogrel achieves more rapid effects than ticlopidine through loading dose administration.18 The stardom of clopidogrel in the clinical settings of PCI and ACS, including unstable angina, non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI), has been undisputed up till now, given that several large-scale clinical trials have shown a clear benefit in terms of preventing recurrent ischemic events, including stent thrombosis, when clopidogrel is associated to aspirin.19-23 In fact, dual antiplatelet therapy with aspirin and clopidogrel is currently accepted per guidelines as the antiplatelet treatment of choice for patients across the spectrum of ACS, including patients with unstable angina, NSTEMI24,25 and STEMI,26,27 as well as for patients undergoing PCI.28,29 Despite these clinical benefits, a substantial number of patients may continue to have recurrent cardiovascular events. Accumulating observations have shown that variability in individual response profiles to clopidogrel has been proposed as one of the mechanisms involved in this limited efficacy.6,7 This has led to investigations trying to identify the mechanisms associated with clopidogrel response variability as well as strategies to overcome the limitations associated with current treatment regimens.30,31
CLOPIDOGREL: INTERINDIVIDUAL VARIABILITY IN RESPONSE
Clopidogrel, like all thienopyridines, is a pro-drug that must undergo hepatic biotransformation to be converted to an active metabolite which will irreversibly bind and block P2Y12 platelet receptor. Approximately 85% of the clopidogrel absorbed into the bloodstream from the intestine is hydrolyzed by esterases becoming inactive, whereas the remaining ≈15% is metabolized in the liver through a double oxidation process mediated by several cytochrome P450 (CYP) isoforms to be converted to an active metabolite.6,7 Due to the irreversible blockade of the P2Y12 receptor by its active metabolite, clopidogrel effects last for the whole lifespan of the platelet (7-10 days).
The delayed onset of action of clopidogrel is one of its limitations. Thus, a loading dose must be administered when rapid inhibition is required, such as in the context of ACS or PCI.18 Currently, the doses approved by regulatory authorities are a 300 mg loading dose and a 75 mg maintenance dose. Given the accumulating evidence of a more rapid and potent effect associated with a 600 mg loading as well as a better clinical benefit, this dosing regimen has now become the standard of care in clinical practice and is also endorsed by practice guidelines.28,32-34 Clopidogrel's main caveat is its broad variability in response among treated individuals. A relatively high percentage of patients experience suboptimal effects; the rate of "low responders" or "resistant patients" ranges from 5% to 40%, depending on population characteristics as well as the platelet function assay and cut-off values used.6,7 Variability in clopidogrel response is a well-known phenomenon the relevance of which is underscored by the fact that a multitude of studies have observed an association between low responsiveness and adverse cardiovascular outcomes.6,7 These studies have been performed mainly in patients undergoing PCI (Table 1), where the use of clopidogrel is mandatory, but also in patients on chronic clopidogrel therapy.35-57
MECHANISMS INVOLVED IN CLOPIDOGREL RESPONSE VARIABILITY
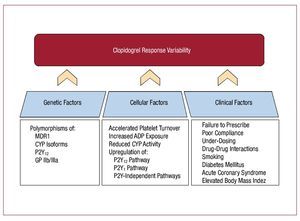
Multiple mechanisms have been identified that play a role in clopidogrel response variability. These can be summarized into 3 broad categories: genetic, cellular, and clinical factors (Figure 2).
Figure 2. Mechanisms involved in clopidogrel response variability Multiple mechanisms are involved in clopidogrel response variability, which can be grouped into three categories: genetic, cellular and clinical factors. ADP indicates adenosine diphosphate; CYP, cytochrome P450; GP, glycoprotein; MDR, multidrug resistance transporter.
Genetic Factors
Pharmacogenetic studies have evaluated polymorphisms of different genes involved in the pharmacokinetic and pharmacodynamic effects of clopidogrel.58 These include genes encoding for proteins and enzymes involved in clopidogrel's absorption and hepatic metabolism as well as genes encoding for platelet membrane receptors.
The gene ABCB1 codifies the intestinal P-glycoprotein MDR1 (multidrug resistance transporter), involved in clopidogrel absorption. Patients carrying two ABCB1 variant alleles may have reduced active metabolite generation after administration of a loading dose of clopidogrel.59 Simon et al observed that the presence of these variant alleles was associated with a higher rate of cardiovascular events (death from any cause, nonfatal stroke and myocardial infarction) at 1 year of follow-up in a population of 2208 patients with an acute myocardial infarction receiving clopidogrel therapy.60 However, the same ABCB1 polymorphism was not found to be associated with ADP-stimulated platelet aggregation after 1 week of clopidogrel therapy in a recently published genome-wide association study performed in an homogenous population (Amish) of healthy subjects.61
A number of CYP isoenzymes are involved in the hepatic oxidation steps that convert clopidogrel to its active metabolite. In particular, CYP3A4, CYP3A5, CYP2C9, and CYP1A2 are implicated in one step, while CYP2B6 and CYP2C19 are involved in both steps. Different experiences have reported polymorphisms in CYP3A4,62 CYP3A5,63 and CYP2C964 to be associated with clopidogrel responsiveness, although large-scale pharmacogenetic studies have failed to observe any association between these polymorphisms and clinical outcomes.60,65 However, a number of recent large-scale studies have showed a strong association between CYP2C19 loss-of-function variant alleles (mainly CYP2C19*2) and impaired clinical outcomes.60,61,65-68 This is in line with numerous studies showing the relation between CYP2C19 reduced-function alleles and decreased formation of active metabolite, lower platelet inhibition and impaired clinical outcomes.64,69-71 In the study by Simon et al, acute myocardial infarction patients carrying any two CYP2C19 loss-of-function alleles (*2,*3,*4, or *5), especially those undergoing PCI, had a higher rate of cardiovascular events at 1 year of follow-up.60 Consistently, a substudy of TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction) showed that carriers of at least one CYP2C19 reduced-function allele had a higher rate of cardiovascular events among clopidogrel-treated subjects (n=1477).65 In addition, the CYP2C19*2 variant has been observed to be an independent predictor of cardiovascular events in patients chronically treated with clopidogrel after a myocardial infarction66 or undergoing PCI,61 as well as to be significantly associated with an increased risk of stent thrombosis following coronary stent placement.67,68
Pharmacogenetic studies have also evaluated polymorphisms of genes encoding for platelet membrane receptors, such as the following: P2YR12 (ADP receptor P2Y12), ITGB3 (platelet-fibrinogen receptor GP IIb/IIIa), ITGA2 (platelet-collagen receptor GP Ia), and PAR-1 (protease-activated receptor -1, a thrombin receptor). Some variants of these genes have been suggested to play a role in variability in clopidogrel response, although results have been inconsistent to date.72-78
Cellular Factors
Clopidogrel-induced antiplatelet effects also may be affected by several cellular factors. For instance, an accelerated platelet turnover has been suggested to diminish clopidogrel responsiveness.79 Platelet turnover is represented by the presence of reticulated (immature) platelets, which could have a greater reactivity and, therefore, result in impaired clopidogrel response. The association between a higher percentage of circulating reticulated platelets and a lower response to clopidogrel has been observed in patients with coronary artery disease, either high-risk79 or stable patients.80 Generation of active metabolite might be affected by cellular factors such as a different degree of baseline metabolic activity of the CYP system.81 In addition, upregulation of both purinergic (P2Y12 and P2Y1) and P2Y-independent platelet signaling pathways have also been proposed to be implicated in clopidogrel variability in response, especially among patients with diabetes mellitus, which may have one or more of these cellular disorders.82-84
Clinical Factors
Multiple factors associated with inadequate clopidogrel response fall into this category. Compliance is the most important.6,7 Clopidogrel dosing may also play a role; whether the currently approved loading and maintenance doses are the most optimal will be discussed later. Some clinical features are also involved in baseline platelet reactivity and response to clopidogrel. In particular, the presence of an acute coronary syndrome,85,86 diabetes mellitus,82,83,87,88 and obesity,89,90 have been associated with lower clopidogrel effects, which may also contribute to higher atherothrombotic event rates.
The CYP system activates and metabolizes countless drugs and substances that could might interfere in hepatic formation of clopidogrel's active metabolite. Some frequently used drugs in cardiovascular therapy that have been suggested to impair clopidogrel-induced antiplatelet effects are lipophilic statins, calcium channel blockers (CCB) and proton pump inhibitors (PPI).
Initially, mechanistic studies observed a relation between the use of lipophilic statins (eg, simvastatin, lovastatin, atorvastatin), which are metabolized by CYP isoenzimes (mainly CYP3A4), and decreased clopidogrel-mediated inhibitory effects.91,92 However, these findings were not corroborated in other functional studies and, importantly, post-hoc analysis of large-scale clinical trials or registries did not show any association with adverse clinical outcomes.93-96 Calcium channel blockers (metabolized by CYP3A4), mainly dihydropyridines, have also been reported to decrease clopidogrel inhibitory effects on platelets and to impair clinical outcomes when both drugs are associated.97,98
A drug-drug interaction between PPIs and clopidogrel has been recently described and has raised an important concern due to the frequency with which these drugs are associated. The different PPIs available are metabolized by CYP isoforms (mainly CYP2C19 and CYP3A4), but with different specificities.99 The most consistent results to date in functional studies have involved omeprazole, which is metabolized primarily by CYP2C19.100,101 In a double-blind, randomized, placebo-controlled study, omeprazole significantly decreased clopidogrel antiplatelet effects in patients (n=124) receiving dual antiplatelet therapy and undergoing coronary artery stent implantation.100 Other PPIs have also been evaluated in functional studies, which failed to show any effect of pantoprazole or esomeprazole on clopidogrel responsiveness,102 while lansoprazole has been reported to reduce antiplatelet effects after a clopidogrel loading dose of 300 mg only in subjects with the higher response (upper tertile), but not in patients receiving a loading dose of prasugrel.103 Data analyses of large clinical studies, mainly registries and post-hoc analysis of randomized clinical trials, have provided contradictory results when evaluating the effect of concomitant therapy with PPIs and clopidogrel on clinical outcomes. Ho et al observed that concurrent PPI and clopidogrel therapy was significantly associated with a 25% relative increase in long-term adverse outcomes (the composite endpoint of death and rehospitalization for ACS) in a cohort of 8205 patients taking clopidogrel after discharge for an ACS.104 PPIs other than omeprazole were rarely used and, hence, the study was underpowered to determine their effects. Global use of PPIs was also found to be a predictor of reinfarction in a population-based case-control study in patients (n=2791) following discharge after treatment for a myocardial infarction. When PPIs were evaluated separately, pantoprazole (metabolized principally by CYP2C9) use was not associated with an increased risk of reinfarction.105 Conversely, results of the Clopidogrel Medco Outcomes study presented during the Society for Cardiovascular Angiography and Interventions (SCAI) 2009 Annual Scientific Sessions (Las Vegas, NV, USA) suggested a class effect. In this large registry (n=16 690), PPIs were associated with increased risk (hazard ratio = 1.51) of cardiovascular events at 12 months of follow-up in patients on clopidogrel following coronary stenting. Each individual PPI (omeprazole, esomeprazole, pantoprazole, and lansoprazole) was associated with a greater risk (39%-61%) of cardiovascular events when compared with clopidogrel alone. However, a post-hoc analysis of the TRITONTIMI 38 trials failed to show any association of PPI use with clinical outcomes in patients on clopidogrel and those on prasugrel therapy, even though a post-hoc analysis of PRINCIPLE-TIMI 44 (Prasugrel in Comparison to clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44) observed that platelet aggregation 6 hours after a 600 mg clopidogrel loading dose was lower for patients on a PPI, while a non significant difference was seen after a 60 mg loading dose of prasugrel.106 These clinical findings are in line with the results of the Clopidogrel and the Optimization of Gastrointestinal Events (COGENT-1) trial, presented at the TCT 2009 meeting (San Francisco, CA, USA. COGENT-1 is the only prospective randomized double-blind placebo controlled trial to date comparing a PPI (omeprazole) with placebo in patients taking clopidogrel. The study enrolled 3627 patients in whom a requirement for clopidogrel therapy with concomitant aspirin was anticipated for at least 12 months. No difference was observed in the risk of cardiovascular events or myocardial infarction (hazard ratio = 1.02; 95% confidence interval, 0.70-1.51) in a median follow-up of 133 days, while a benefit in terms of reduced gastrointestinal effects, which was the primary outcome of the study, was seen in patients taking the PPI (hazard ratio = 0.55; P<.007).
Smoking is a major risk factor for atherothrombotic cardiovascular processes and smoking cessation is a class I recommendation for secondary prevention of ischemic events in patients with coronary artery disease.24-29 Cigarette smoking is also a potent inducer of the CYP1A2 isoform107 and, therefore, it may increase clopidogrel biotransformation. Some recent studies have reported that a heavy smoking habit enhances clopidogrel-induced inhibitory effects on platelets108,109 and improves clinical outcomes in clopidogrel-treated patients.110,111 However, a mechanistic study observed an association between cigarette smoking and a lower production of one of clopidogrel's metabolites.112 Therefore, the role of smoking on clopidogrel effects warrants further investigation.
FUTURE DIRECTIONS
The prognostic implications associated with variability in clopidogrel-induced effects inevitably lead to questions on how to address and overcome this phenomenon. Essential first steps are to confirm patient compliance to antiplatelet treatment and rule out potential drug-drug interactions in the polymedicated patient. Three additional strategies have been suggested to overcome variability in response to clopidogrel6,7:
High Clopidogrel Dosing
A high clopidogrel loading dose of 600 mg achieves faster and greater platelet inhibition than the current standard of 300 mg,32,113,114 while a 900 mg loading dose provides only a marginal increase in platelet inhibition when compared to a 600 mg loading dose.113,114 This greater platelet inhibition with high clopidogrel loading regimens has been reflected in better clinical outcomes in patients undergoing PCI and has become common clinical practice despite the lower current standard..33,34,115
In a PCI setting, randomized experiences have observed a benefit of a high maintenance regimen (150 mg/day) of clopidogrel in terms of enhanced platelet inhibition when compared to the standard dose of 75 mg/day.116-119 In a large observational study performed in a nonselected cohort of patients (n=2954) who underwent PCI with coronary stenting, Lemesle and colleagues compared the effect of a high loading dose followed by a high maintenance dose (600 mg and 150 mg/day, respectively) of clopidogrel with standard dosing during the first 15 days after PCI. In this registry, the high dosing regimen was significantly associated with a decrease in the composite end point of death, myocardial infarction and stent thrombosis (hazard ratio = 0.694) at 2 months without a significant increase in hemorrhagic complications.120 These findings are in line with the results of the CURRENT/ OASIS-7 (Clopidogrel optimal loading dose Usage to Reduce recurrent EveNTs/Optimal Antiplatelet Strategy for InterventionS; European Society of Cardiology Congress 2009, Barcelona, Spain). This multicenter, randomized, parallel-group trial enrolled 25 087 ACS patients scheduled to undergo angiography within 72 hours of hospital arrival who were randomized to high dose (600 mg of clopidogrel on the first day, then 150 mg once a day for 7 days, followed by 75 mg daily for the remainder of the month) or standard dose of clopidogrel for a month. This study had a 2´2 factorial design and patients were also randomized to receive high (300-325 mg daily) versus low (75-100 mg daily) dose of aspirin. Although the study did not find a statistical difference for the primary endpoint (the combined rate of cardiovascular death, myocardial infarction and stroke at 30 days) in the overall study population, the high clopidogrel dose regimen reduced the risk of stent thrombosis by 30% and the risk of myocardial infarction by 22% in the subgroup of patients undergoing PCI (n=17 232), while no benefit was observed in patients who did not undergo PCI. The benefit observed in the PCI subgroup was, however, hampered by an increase in major bleeding in the high dose regimen group, although it was not significant for intracerebral or fatal bleeds. No significant difference in efficacy or bleeding between high and low-dose aspirin was observed, although a trend towards a higher rate of gastrointestinal bleeds in the high-dose group (0.38% vs 0.24%; P=.051) was found.
There has also been emerging interest in increasing clopidogrel dosing based on the degree of responsiveness of a given patient, which has been defined as "tailored" or "individualized" treatment. Bonello et al observed that additional 600 mg loading doses of clopidogrel (up to 2400 mg) administered to low-responders ("tailored" treatment) reduced the rates of adverse events, including stent thrombosis, compared to patients treated conventionally without increasing the bleeding risk.121,122 The efficacy and safety of tailored treatment with high clopidogrel maintenance dose in low responders to standard clopidogrel dose is currently under evaluation in several ongoing clinical trials, such as GRAVITAS (Gauging Responsiveness With a VerifyNow Assay: Impact on Thrombosis and Safety; NCT00645918),123 ARCTIC (Double Randomization of a Monitoring Adjusted Antiplatelet Treatment Versus a Common Antiplatelet Treatment for DES Implantation, and Interruption Versus Continuation of Double Antiplatelet Therapy; NCT00827411), and DANTE (Dual Antiplatelet Therapy Tailored on the Extent of Platelet Inhibition, NCT00774475).
Triple Antiplatelet Therapy
Adding a third antiplatelet drug may be considered as an option both in the acute and maintenance phases of treatment. Glycoprotein IIb/IIIa inhibitors may be used in the acute phase, as they markedly increase platelet inhibition when added on top of clopidogrel.44 Studies evaluating tailored treatment according to the degree of responsiveness to standard antiplatelet therapy have obtained promising results. In a cohort of clopidogrel low responder patients (n=149) referred for elective PCI who were randomized to "conventional group" (standard dual antiplatelet therapy) or "active group" (addition of abciximab to dual antiplatelet therapy), Cuisset et al observed that patients in the active group had a significantly lower rate of cardiovascular events at 1 month (OR=2.8).124 The recently published 3T/2R (Tailoring Treatment With Tirofiban in Patients Showing Resistance to Aspirin and/or Resistance to Clopidogrel) trial randomized stable or low-risk unstable angina patients undergoing elective PCI who were poor responders (n=263) to aspirin or clopidogrel to receive either tirofiban (n=132) or placebo (n=131) on top of standard aspirin and clopidogrel therapy. The rate of major adverse cardiovascular events within 30 days was reduced in the tirofiban group (3.8% vs 10.7%), without any increased risk in bleeding.125
In the maintenance phase of therapy, adjunctive use of cilostazol to standard dual antiplatelet therapy has been observed to increase the degree of platelet inhibition.126 The enhanced platelet inhibition achieved with this triple therapy may contribute to the observed association with better clinical outcomes in patients undergoing PCI, including stent thrombosis rates.127-129 Of note, this benefit seems not to be hampered by an increase in bleeding.127 However, use of cilostazol is limited by the high frequency of side effects, mainly headache, palpitations, and gastrointestinal disturbances.126
New P2Y12 Receptor Antagonists
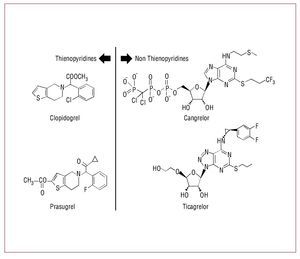
The benefit achieved by blocking the P2Y12 signaling pathway in patients with coronary artery disease for preventing recurrent events is indisputable. Thus, the search for new agents with higher inhibitory effects and less variability compared to clopidogrel is warranted (Figure 3). Currently, several novel P2Y12 blockers are under different stages of clinical development130,131 (Table 2). This section aims to provide an overview of these new agents.
Figure 3. Chemical structure of P2Y12 receptor antagonists.
Prasugrel
Prasugrel, a third-generation thienopyridine, is an orally administered pro-drug which needs hepatic biotransformation into its active metabolite to irreversibly block the P2Y12 receptor.132 The major pharmacokinetic difference with clopidogrel is that prasugrel is more effectively converted to its active metabolite, through a process involving hydrolysis by carboxyesterases, mainly in the intestine, followed by only a single hepatic CYP-dependent step. Since the active metabolites of clopidogrel and prasugrel are equipotent in terms of platelet inhibitions, the major production of active metabolites achieved by prasugrel provides greater platelet inhibition.132 In addition, prasugrel has a more rapid onset of action and less interindividual response variability than clopidogrel even when used at high dosing regimens.132,133
The TRITON-TIMI 38 trial evaluated the clinical efficacy and safety of prasugrel (60 mg loading dose followed by a 10 mg maintenance dose), compared to standard clopidogrel loading and maintenance dose regimens in 13 608 patients with moderate to high-risk ACS undergoing PCI.134 In this randomized, double-blind, parallel-group, phase III study, prasugrel obtained a significant 19% relative reduction (9.9% for prasugrel vs 12.1% for clopidogrel; hazard ratio = 0.81; P<.001) of the rates of the primary end point (composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke), and a significant reduction of the rates of stent thrombosis (9.7% vs 11.9%; hazard ratio = 0.81; P=.0001),135 over a follow-up period of 15 months. This occurred at the cost of an increased risk of TIMI major non-coronary artery bypass grafting (non-CABG) related bleeding (2.4% vs 1.8%; P=.03), mostly in the maintenance phase of prasugrel treatment.134 An important feature of this trial is the performance of a net clinical benefit analysis (a composite of the efficacy and bleeding end points), in which prasugrel was still found superior despite the excess in bleeding (12.2% vs 13.9%; hazard ratio = 0.87; P=.004). The clinical benefit of prasugrel was largely driven by a marked reduction in non-fatal MI, while no differences were observed in death and stroke. Particular subgroups appeared to benefit more from the use of prasugrel, such as patients with diabetes mellitus136 and patients with STEMI,137 in which no increase in bleeding risk was observed. In contrast, the net analysis mentioned above showed no net benefit in the aged patients (≥75 years) and in those weighing less than 60 kg, and a net harm in patients with history of stroke or transient ischemic attack.134 A landmark analysis of this trial showed a significant reduction in ischemic events in the prasugrel group by the third day and persisting throughout the follow-up period.138 Importantly, this analysis suggests a continued clinical benefit of achieving greater platelet inhibition during the maintenance phase of therapy.
Prasugrel has been recently approved for clinical use by regulatory authorities, but only in the setting of ACS patients undergoing PCI. The clinical efficacy of prasugrel in medically managed patients with unstable angina/NSTEMI is currently being evaluated in the TRILOGY-ACS (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes) trial (NCT00699998).
Ticagrelor
Ticagrelor is an orally administered cyclopentyltriazolopyrimidine, which directly and reversibly inhibits the platelet P2Y12 receptor.132,139 Its pharmacokinetic and pharmacodynamic properties include: a) rapid absorption and onset of action; b) higher inhibition of platelet aggregation than clopidogrel; and c) rapid offset of action, as it has a half-life of 12 hours (requires twice daily dosing).140,141 The recently published PLATO (Platelet Inhibition and Patient Outcomes) trial evaluated the benefit of ticagrelor (180 mg loading dose followed by 90 mg twice daily) compared to clopidogrel (300 to 600 mg loading dose followed by 75 mg daily) in preventing cardiovascular events in 18 624 patients with an acute coronary syndrome, with or without ST-segment elevation.142 In this trial, ticagrelor therapy significantly reduced the rate of the primary endpoint (death from vascular causes, myocardial infarction or stroke) at 12 months (12.3% vs 10.2%; hazard ratio =0.84; P=.0001) and, remarkably, the rate of cardiovascular death (4.0% vs 5.1%; P=.001), death from any cause (4.5% vs 5.9%; P<.001) and definite or probable stent thrombosis (2.2% vs 2.9%; P=.02) in the subgroup of patients undergoing PCI. Although no increase in major bleeding was found using the protocol definition (11.6% vs 11.2%; P=.43), ticagrelor was associated with a higher rate of major bleeding not related to coronary-artery bypass grafting (4.5% vs 3.8%; P=.03). Under the TIMI major non-CABG related bleeding definition used in the TRITON trial, there was a similar increase in the rate of bleeding with ticagrelor (2.8% vs 2.2%; P=.03). In addition, non-bleeding safety concerns were noted. Dyspnea was more frequent in the ticagrelor group (13.8% vs 7.8%; P<.001), which led to a significant rate of treatment discontinuation compared to clopidogrel (0.9% vs 0.1%; P<.001). Also, patients in the ticagrelor group presented a significantly higher increase in creatinine and uric acid from baseline than those in clopidogrel group at 1 and 12 months (P<.001 for both), as well as a higher percentage of ventricular pauses (≥3 seconds) in the first week (P=.01), although no difference in bradycardia-related events was found. These non-bleeding side effects are likely attributed to off-target effects of ticagrelor or its metabolites.
Cangrelor
Cangrelor is an intravenous ATP analog which reversibly and directly, without any biotransformation, inhibits the P2Y12 receptor.132,139 The main pharmacokinetic and pharmacodynamic properties of cangrelor are: a) rapid onset of action, reaching steady-state concentrations within minutes; b) great degree of platelet inhibition (>90%); c) dose-dependent effects; and d) rapid offset of action, since it has an extremely short half-life (2-5 minutes) due to rapid deactivation by plasmatic ectonucleotidases.143,144 In spite of the promising results obtained in phase II studies, which showed cangrelor to be a potent platelet inhibitor with a relatively safe profile,143,144 these findings have not been corroborated in phase III studies. The CHAMPION (Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition) program included the recently published CHAMPION-PCI (n=8716)145 and the CHAMPION-PLATFORM146 (n=5362) trials, which have been recently published. These studies aimed to evaluate the efficacy of cangrelor in patients, most with ACS, undergoing PCI. Cangrelor was not found superior for reducing the primary end point, a composite of death from any cause, MI, or ischemia-driven revascularization at 48 hours, when compared to clopidogrel in the CHAMPION-PCI study (7.5% vs 7.1% (OR=1.05 [0.88-1.24]; p=0.56) and compared to placebo in CHAMPION-PLATFORM (7.0% vs. 8.0%; OR=0.87 [0.71-1.07]; p=0.17). However, the pharmacological properties of cangrelor make this a promising drug in the setting of patients requiring surgery who need a bridging antiplatelet strategy. This is a current objective of the ongoing BRIDGE (maintenance of platelet inihiBition with cangRelor after dIscontinuation of thienopyriDines in patients undergoing surGEry) trial (NCT 00767507).
Elinogrel
Elinogrel is a novel, direct-acting, and reversible P2Y12 inhibitor which can be administered both orally and intravenously.147 Elinogrel is currently in the preliminary stages of development, but phase I studies have shown interesting pharmacologic properties: a) rapid onset of action (almost immediate if administered intravenously); b) higher degree of platelet inhibition than clopidogrel; and c) rapid offset of action, being its half-life of 50 minutes and 12 hours for intravenously and oral administration, respectively.147 Results from a pharmacodynamic study were presented at the American Heart Association Congress 2008 (New Orleans, LA, USA), showing that a single oral dose of elinogrel improved platelet inhibition in stable patients with coronary artery disease that were poor clopidogrel responders.148 Currently, the ongoing INNOVATE (a Randomized, Double-Blind, Active-Controlled Trial to Evaluate Intravenous and Oral PRT060128, a Selective and Reversible P2Y12 Inhibitor, vs Clopidogrel, as a Novel Antiplatelet Therapy in Patients Undergoing Non-Urgent PCI) trial (NCT00751231) is evaluating clinical efficacy, biological activity, tolerability and safety of PRT060128 in patients undergoing non-urgent PCI, testing three doses of elinogrel (oral 50, 100, and 150 mg) twice daily, following an intravenous bolus.
CONCLUSIONS
Platelet P2Y12 receptor antagonism with clopidogrel has represented a major advancement in the treatment of patients with atherothrombotic disease, in particular those with ACS and those undergoing PCI. Despite the clear clinical benefit associated with clopidogrel in these patients, laboratory and clinical experience have helped to identify some caveats, among which its broad platelet inhibitory response profile is the most relevant. Genetic, cellular and clinical factors are implicated in variability in response to clopidogrel, which has shown to be associated with adverse clinical outcomes. Therefore, the search for new strategies to optimize platelet inhibition is strongly warranted. Indeed, the development of new P2Y12 receptor blockers with more favorable pharmacokinetic and pharmacodynamic profiles represent an important step forward in this field. Evaluation of recently reported large-scale trials and the upcoming results of ongoing clinical investigations will provide the bases for a future of individualized and more specific antiplatelet treatment regimens.
ACKNOWLEDGMENTS
We are grateful to Mariana Muñoz, MD, for her assistance in figure preparation.
Disclosures: Dominick J. Angiolillo:
Honoraria/Lectures: Bristol Myers Squibb; Sanofi-aventis; Eli Lilly and Company; Daiichi Sankyo, Inc.
Honoraria/Advisory board: Bristol Myers Squibb; Sanofi-aventis; Eli Lilly and Company; Daiichi Sankyo, Inc.; Astra Zeneca; The Medicines Company; Portola Pharmaceuticals; Novartis; Arena Pharmaceuticals. Research Grants: GlaxoSmithKline; Otsuka; Accumetrics; Eli Lilly and Company; Daiichi Sankyo, Inc.; The Medicines Company; AstraZeneca; Eisai; Portola Pharmaceutical; Schering-Plough; Johnson and Johnson.
Correspondence: D.J. Angiolillo, MD, PhD,
University of Florida College of Medicine-Jacksonville, 655 West 8th Street, Jacksonville, Florida, 32209, USA
E-mail: dominick.angiolillo@jax.ufl.edu