The heart is forever making the head its fool.
Ever since the World Health Organization's report of 1979,1 the diagnostic criteria for myocardial infarction (MI) have remained in a state of evolution. Over the years, increasing emphasis has been placed on biochemical indicators of myocardial necrosis—initially creatinine kinase-isoenzyme MB (CK-MB) and latterly cardiac troponin (cTn)—whilst technological advances have seen ever more sensitive assays introduced into clinical practice. In suspected acute coronary syndrome, the evidence is clear that even small increases in cTn above the 99th centile upper reference limit (URL) have important diagnostic and prognostic implications.2,3 Consequently, the 99th centile has become the recommended diagnostic threshold for spontaneous, or type 1, MI.4
A similar prognostic association has been assumed in the setting of myocardial necrosis following percutaneous coronary intervention (PCI) where the mechanism of biomarker release may relate to recognized or occult complications including coronary dissection, stent thrombosis, side-branch occlusion, vascular spasm, or atherothrombotic embolization. At first glance, such an assumption seems legitimate; early experiences with angioplasty were associated with significant rates of these acute coronary complications, and such events were important causes of morbidity and mortality.5 A number of studies reported that an increase in CK-MB concentration following angioplasty was associated with long-term mortality, even in the absence of peri-procedural ischemic symptoms or changes in the electrocardiogram.6 However, the inclusion of patients with both acute and stable coronary artery disease, incomplete adjustment for baseline clinical characteristics, and differences in the assay and diagnostic threshold have prevented definitive conclusions as to the clinical implications of myocardial necrosis following coronary intervention.
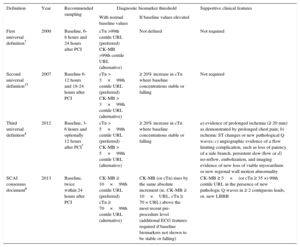
Ignoring this uncertainty, a consensus document published in 2000 by a joint European Society of Cardiology/American College of Cardiology (ESC/ACC) committee encouraged routine measurement of cTn before and after PCI and recommended that the same diagnostic threshold—the 99th centile URL—be employed for both spontaneous and procedural MI.7 A host of subsequent studies revealed procedure-related increases in cTn concentration to be common, occurring in up to a third of cases, but frequently failed to find a clear link between small increases and long-term outcomes,8–12 although larger increases, particularly of CK-MB, were held to be more discriminatory.13,14 The guidelines were revised in light of this evidence. Whilst acknowledging the arbitrary nature of any threshold, the diagnosis of procedural or type 4a MI now requires cTn concentrations ≥ 5×the URL in patients with concentrations below the URL prior to the procedure (Table 1). Critically, the guidelines now require additional supplementary criteria including symptoms, electrocardiogram changes or imaging evidence of infarction to make a diagnosis of procedural MI.4 Disagreement persists, however, with opposition most notably led by the Society for Cardiovascular Angiography and Interventions (SCAI), who propose that the fault lies in the biomarker thresholds chosen. Their recently published diagnostic definitions obligate a rise in CK-MB to ≥ 10×URL, or cTn to ≥ 70×the URL, with no requirement for additional clinical evidence of myocardial ischemia or infarction.6 Here, the measurement of CK-MB is favored over cTn and the threshold is halved where new pathological Q waves develop following the procedure. As with the ESC/ACC guideline, the thresholds only apply when the baseline biomarkers are normal.
Evolving Definitions of Perirocedural Myocardial Infarction
| Definition | Year | Recommended sampling | Diagnostic biomarker threshold | Supportive clinical features | |
|---|---|---|---|---|---|
| With normal baseline values | If baseline values elevated | ||||
| First universal definition7 | 2000 | Baseline, 6-8 hours and 24 hours after PCI | cTn >99th centile URL (preferred) CK-MB >99th centile URL (alternative) | Not defined | Not required |
| Second universal definition15 | 2007 | Baseline 6-12 hours and 18-24 hours after PCI | cTn > 3×99th centile URL (preferred) CK-MB > 3×99th centile URL (alternative) | ≥ 20% increase in cTn where baseline concentrations stable or falling | Not required |
| Third universal definition4 | 2012 | Baseline, 3-6 hours and optionally 12 hours after PCI* | cTn > 5×99th centile URL (preferred) CK-MB > 5×99th centile URL (alternative) | ≥ 20% increase in cTn where baseline concentrations stable or falling | a) evidence of prolonged ischemia (≥ 20 min) as demonstrated by prolonged chest pain; b) ischemic ST changes or new pathological Q waves; c) angiographic evidence of a flow limiting complication, such as loss of patency of a side branch, persistent slow-flow or d) no-reflow, embolization, and imaging evidence of new loss of viable myocardium or new regional wall motion abnormality |
| SCAI consensus document6 | 2013 | Baseline, twice within 24 hours after PCI | CK-MB ≥ 10×99th centile URL (preferred) cTn ≥ 70×99th centile URL (alternative) | CK-MB (or cTn) rises by the same absolute increment (ie, CK-MB ≥ 10×URL, cTn ≥ 70 × URL) above the most recent pre-procedure level (additional ECG features required if baseline biomarkers not shown to be stable or falling) | CK-MB ≥ 5×(or cTn ≥ 35 ×) 99th centile URL in the presence of new pathologic Q waves in ≥ 2 contiguous leads, or, new LBBB |
CK-MB, creatinine kinase-MB isoenzyme; cTn, cardiac-specific troponin; ECG, electrocardiogram; LBBB, left bundle branch block; MI, myocardial infarction, PCI, percutaneous coronary intervention; SCAI, Society for Cardiovascular Angiography and Intervention; URL, upper reference limit.
It is in the context of this debate that, in their article published in Revista Española de Cardiología, Ndrepepa et al16 performed a retrospective analysis of 3463 patients undergoing elective PCI for stable coronary artery disease to ascertain the prognostic significance of procedural increases in cTn. They employed the high-sensitivity cTnT (hsTnT) assay with a lower limit of detection of 5.0 ng/L and a 99th centile URL of 14ng/L. All patients underwent blood sampling prior to the procedure with subsequent serial measurements at 6, 12, and 24hours. The primary outcome was all-cause mortality with a median follow-up of around 15 months. In total, almost 80% of the cohort were found to have peak cTnT concentrations above the URL. Three groups were compared, stratified by peak troponin T concentration: group 1 with hsTnT ≤ URL (n = 742; 21.4%), group 2 with hsTnT > URL but ≤ 5 v URL (n = 1928; 55.7%), and group 3 with hsTnT > 5×URL (n = 793; 22.9%). Perhaps unsurprisingly, univariate analysis identified a correlation between postprocedural hsTnT and increased mortality. Importantly however, coronary disease burden and procedural complexity were strong predictors of postprocedural increases in cTnT concentration, and on multivariate analysis, adjusting for these confounding factors procedural increases in cTnT concentration did not predict mortality.
Several limitations of this study should be noted. By design, this was a low risk population, and, with a correspondingly small number of events (56 deaths, 1.6%, at a median follow-up of 15 months) it was underpowered for the number of variables included in the regression models. Further increasing the risk of a type II error, although presumably with the intention of minimizing the risk of bias, Ndrepepa et al reported all-cause mortality rather than cardiovascular mortality. It is unlikely that procedure-related increases in cTn concentration would predict deaths from noncardiovascular conditions, as such it would have been useful to provide univariate estimates for both all-cause and cardiovascular mortality. Another weakness is the potential for clinicians to introduce treatment bias, assuming cTn concentrations were available contemporaneously, whereby patients with increased cTn concentrations were managed differently from those without. Finally, although by definition patients in group 3 met the current biochemical criteria for type 4a MI, it is unclear how many of these individuals fulfilled the additional requirements for this diagnosis. Presumably, this was a relatively infrequent event given that the incidence of post-PCI TIMI flow grade ≤ 2 was only 2% and the development of new Q waves was seen in 0.2%. Nevertheless, this omission makes it challenging to draw robust conclusions concerning the clinical validity of the current guidelines.
Notwithstanding these caveats, the authors should be commended on a number of strengths in their report. Firstly, it is one of the first studies to address this issue using a high-sensitivity cTn assay. This is of particular merit in light of the growing body of evidence that cTn concentrations prior to PCI have prognostic value independent of other clinical and procedural variables. By using an assay that has enhanced precision at the URL they were able to accurately define the population with normal baseline values. Similarly, by requiring that patients had stable symptoms for at least 2 months prior to the procedure, they avoided the pitfall of some earlier studies that may have included unstable patients even though their biomarker concentrations prior to the procedure appeared to be within the normal range. In these patients, troponin concentrations may have been above the URL if a high-sensitivity assay had been used, or cTn concentrations may not yet have peaked.17 Finally, by performing a multivariate analysis adjusting for baseline demographic, anatomic and procedural characteristics with known prognostic significance (Table 2), they have avoided the logical fallacy of post hoc ergo propter hoc.
Predictors of Peri-procedural Myocardial Infarction
| Baseline clinical features | Atherosclerotic burden | Procedural |
|---|---|---|
| Advanced age Acute coronary syndrome Left ventricular dysfunction Smoking history Prior myocardial infarction | Multi-vessel disease Visible thrombus Long lesions Bifurcating lesions Chronic total occlusions | Prolonged procedure Increased contrast volume Side branch occlusion Multiple stents Distal embolization/no reflow Rotational atherectomy Vein graft intervention |
How should these findings be interpreted in light of prior research? In brief, we believe the observations from the present study are consistent with the majority of earlier studies despite some apparent discrepancies. Many previous reports support the conclusion that small increases in cTn concentrations following PCI are common and have negligible independent prognostic value.9,11 Where investigators have published results that contradict this, the studies have not rigorously excluded individuals with elevated, or potentially still rising, baseline concentrations.18,19 It is clear that pre-procedural myocardial injury is a much more powerful prognostic marker, and in these patients it is not possible to definitively attribute a subsequent increase in biomarker concentration as peri-procedural.8 In the remainder of studies linking elevated biomarkers with poor long-term outcomes, there has been no adjustment for the confounding characteristics of baseline risk, disease burden, and procedural complexity.20–23 Ultimately, however, we would add that the present report does not exclude the potential prognostic value of more marked increases in cTn concentration, such as those above the thresholds advocated in the SCAI guidelines, and if procedural complications are suspected, biomarker ascertainment remains a vital diagnostic tool. Outside this high-risk setting, however, there appears to be no benefit in routinely identifying peri-procedural myocardial injury.
The findings of the present study, in combination with existing evidence has some valuable potential implications.
- 1.
Post-procedural cTn concentration is not a reliable indicator of the quality of care for PCI. As noted in the SCAI consensus document, the introduction of more sensitive biomarker assays has increased the incidence in type 4a MI despite overall improvements in PCI outcomes.6 Given the heterogeneity of patient populations and inconsistent recording of both pre- and postprocedural biomarkers, attempting to use these measures as an indicator of quality is inappropriate. Perhaps in recognition of this, the current ESC guidelines on myocardial revascularization have removed the earlier recommendation for routine testing after PCI.24
- 2.
Clinical trials incorporating procedural MI in composite endpoints should be interpreted with caution. This is essential given the frequency of small changes in cardiac biomarkers following PCI, and the likelihood this will obscure the true impact of a novel therapy on more clinically meaningful outcomes. In situations where there remains value in reporting such events, it is imperative that standardized criteria are applied and explicitly described. The 2 FAME trials—the first comparing the use of fractional flow reserve (FFR) with conventional angiography for guiding PCI, the second comparing FFR-guided PCI with optimal medical therapy (OMT)—provide an illustrative example in this regard.25,26 Each took place in the setting of clinically stable coronary artery disease and had primary endpoints comprising a composite of death, MI (including type 1 and type 4a) and revascularization, and both trials demonstrated reductions in this combined endpoint with FFR-guided revascularization. However, while both trials mandated post-PCI biomarker sampling to detect procedural MI, the diagnostic cutpoints differed substantially. In FAME-1, spontaneous and procedural MI were identically defined as an elevation of CK-MB ≥ 3×URL; procedural MI contributed around a third of total events and there was little difference between the treatment groups. In contrast, FAME-2 adopted divergent diagnostic criteria with a type 4a MI requiring a 10-fold increase in CK-MB, whilst type 1 could be determined by either CK-MB or troponin using the URL as the diagnostic threshold. The initial analysis showed no overall difference in rates of MI, but a subsequent landmark analysis demonstrated that whereas rates of MI were predictably increased in the PCI group within 7 days of randomization, this pattern was reversed over the subsequent 2 years. Conversely, had FAME-2 retained the less stringent threshold of the earlier trial, it is wholly conceivable that the resulting increased incidence of type 4a MI would have been of sufficient magnitude to eradicate any difference in the primary endpoint. Discrepancies in the diagnostic classification of procedural MI is highly likely to influence current and future clinical trial outcomes.
- 3.
Peri-procedural myocardial injury is not equivalent to spontaneous MI. Firstly, the extent of myocardial necrosis is typically minimal in the post-PCI setting, and on its own is unlikely to compromise ventricular function. Furthermore, a critical driver of recurrent events following type 1 MI is the presence of a persistent and systemic atherogenic process; a mechanism that has little relation to the isolated insult responsible for type 4a events. These concepts were explored by Bangalore et al,27 in a large meta-analysis of 12 trials comparing PCI with OMT in stable coronary artery disease with 37548 patient-years of follow-up. They found that PCI compared with OMT alone was associated with a significant reduction in rates of spontaneous type 1 at the cost of increased procedural events, with no overall difference in the incidence of all MI. Most intriguingly, the point estimate for mortality paralleled the reduction in spontaneous and not procedural MI. Notwithstanding the adage that correlation does not signify causation, such a finding lends further justification to our belief that type 1 and type 4a MI are of differing clinical relevance.
As always in clinical research, unanswered questions persist: would the prognostic value of postprocedural cTn improve if a higher diagnostic threshold was adopted; given the uncertain prognostic significance of type 4a MI, how should such a diagnosis influence therapeutic decisions; is it possible to make this diagnosis in the setting of acute coronary syndrome; should preprocedural troponin concentrations be routinely measured and what are the clinical implications when elevated?
With regards to the question of thresholds, some investigators have indeed reported incremental hazard at progressively higher biomarker concentrations.14,18 Evidence in support of this comes from a study which identified an optimal diagnostic cutpoint for cTn of 112.5×URL (or CK-MB levels above 2.6×URL) when late gadolinium enhancement on cardiac magnetic resonance imaging was used as the determinant of peri-procedural MI.28 It might reasonably be argued that any MI too small to be identified on cardiac magnetic resonance is too small to be of clinical relevance and this appears the view adopted by SCAI. In countering this argument, however, a meta-analysis of 6 stent trials observed that even large increases in CK-MB (> 8×URL) did not predict mortality in the absence of a recognized procedural complication.29 It should be acknowledged that any threshold chosen is arbitrary in nature, and it remains our belief that type 4a MI, by reflecting a transient insult in contrast to the ongoing process of systemic inflammation and plaque vulnerability, has little in common with spontaneous type 1 MI.
The concept of peri-procedural MI has evolved significantly over the past 2 decades, but diagnostic thresholds continue to be arbitrary and open to dispute. Ndrepepa et al have added fuel to the debate concerning whether such a diagnosis is clinically meaningful or is simply an indicator of anatomic and procedural complexity. Addressing these uncertainties is of importance given their potential impact on clinical trial outcomes and on measures of quality of care. Embracing the insight afforded by high-sensitivity cTn assays with the intention of reducing PCI-related morbidity and mortality is clearly an admirable objective. Ultimately, however, more work is required before we conclude that measuring the heart's “temperature” will help us address this fever.
FUNDINGN.L. Mills is supported by a Senior Clinical Research Fellowship from the British Heart Foundation (FS/16/4/32023). P.D. Adamson is supported by the Edinburgh and Lothians Health Foundation (50-534).
CONFLICTS OF INTERESTN.L. Mills has acted as a consultant for Abbott Laboratories, Roche, Singulex and Beckman-Coulter.