Keywords
INTRODUCTION
Due to the aging of the population and the use of new and increasingly effective imaging techniques, the prevalence of thoracic aorta diseases has increased significantly in recent years. On the other hand, knowledge concerning the natural history of these processes has helped establish new therapeutic procedures. Periodic follow-up and progress in medical treatment have improved survival in these patients but, despite this, the progression of the disease cannot be stopped. This means that treatment is needed to prevent the expansion and rupture of the aorta. Until now, surgery was the only therapeutic option available, but it has high rates of morbidity and mortality. Endovascular stent-grafting is a less invasive alternative to surgery and can be used even in patients who, due to age or other associated diseases, are not currently considered suitable for surgical treatment.1-6
The aim of this study is to present and evaluate our experience of endovascular stent-grafting in thoracic aorta diseases and also describe a collaboration project between different medical and surgical specialties.
PATIENTS AND METHODS
Thoracic Aorta Unit of the Central University Hospital of Asturias
A multidisciplinary unit was created in 2001 in the Central University Hospital of Asturias in order to appropriately respond to the diagnosis of thoracic aorta disease and the various forms of treatment that can be applied to it. The unit was made up of 1 cardiac surgeon, 1 vascular surgeon, 1 vascular radiologist, 1 anesthesiologist, 1 cardiologist from the hemodynamics and cardiology unit, and 1 from echocardiography. All the patients with thoracic aorta disease are evaluated in this unit and the most suitable therapeutic approach chosen for each patient.
Patients
Between December 2001 and January 2004, 15 patients from the unit were selected for percutaneous intraluminal treatment. Patient characteristics and the reasons for implantation are shown in Tables 1 and 2.
Study Protocol Prior to Treatment
After clinical evaluation the following studies were carried out in all the patients:
Computerized Tomography
Angiography was done via helical computerized tomography (CT) with sections 3-5-mm thick and at intervals of 2-3 mm. Evaluation of the thoracic aorta included at least the area from the supra-aortic branches to the renal arteries. The basic data sought via CT were as follows:
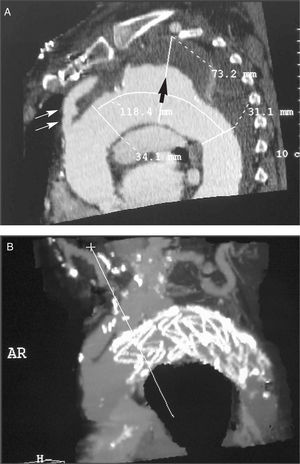
- In the aneurysm: its diameter and length. Diameter and state of the aorta (presence of thrombus and/or calcium) in the expected proximal and distal placement site of the stent. Distance of the aneurysm to the left subclavian artery and to the celiac artery (Figure 1 A and B).
Figure 1. A: reconstruction of the computerized tomography prior to intervention in a patient with aneurysm in the thoracic aorta that included the origin of the left subclavian artery. Proximal diameter (34.1 mm) and distal diameter (31.1 mm); aneurysm (73.2 mm) filled with a large mural thrombus. Large arrow: mural thrombus. Double arrow: bypass from the ascending aorta to the supra-aortic branch. B: the same patient, after the stent was implanted.
- In the dissections: relationship of the beginning of the dissection to the origin of the supra-aortic branches, looking for communication between the two lumens. The relationship of the dissected intima to the origins of the visceral branches and iliac arteries was also determined, as well as the state of the abdominal aorta and the diameters of the 2 lumens.
Arteriography
If the patient was accepted for endovascular stent-grafting after CT, an arteriography was carried out with a catheter marked in centimeters. During arteriography it was considered basic to:
- Define the relationship of the lesion to be treated with the celiac artery.
- Study the iliofemoral access route.
- Estimate the dimensions of the stent to be implemented.
Magnetic Resonance Imaging (MR)
Magnetic resonance was done in the following cases:
- When there were doubts concerning the relationship between the aneurysm and the supra-aortic branches.
- In complex dissections.
Intervention Procedure
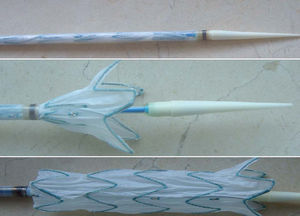
Before the intervention informed consent was obtained from each patient as approved by the center's Ethics Committee. The Talent® (Medtronic, Sunrise, Fl) stent was used (Figure 2). This is a straight self-expanding stent made up of a polyester prosthesis and parallel nitinol rings linked by a connecting bar. For delivery the stent is mounted on a catheter and compressed in a sheath. The external diameter of the system varies between 22 Fr and 25 Fr. In all cases we used a stent whose proximal and distal diameter was 10% larger than the diameters proximal and distal to the lesion, as measured by previous computed tomographic angiography.
Figure 2. TALENT® thoracic stent. Above: stent within the carrier. Center: first part of the stent open. Below: the fully opened stent.
Two patients needed vascular surgery in the supra-aortic branches before implantation. The first patient had undergone surgery several years earlier for acute dissection of the ascending aorta. The dissection had progressed from the aortic arch and the descending aorta towards the iliac bifurcation. The second patient showed an aneurysm of the aortic arch and descending aorta that included the origin of the left subclavian and left carotid arteries. In both cases we implanted an aorto-bilateral carotid bypass with ligature of the innominate artery and the carotid and left subclavian arteries to permit the later delivery of the stent from the upper third of the ascending aorta.
All the procedures were carried out in the hemodynamics laboratory under general anesthesia and all were scheduled, except for 4:1 traumatic rupture of the aorta, 2 acute type B dissections with visceral involvement and 1 ruptured saccular aneurysm with mediastinal hematoma. We used the femoral access route by means of dissection. Whenever possible the right femoral artery was selected, since delivery is easier when the curves are in the same direction. A percutaneous 6 Fr pigtail catheter was introduced through the contralateral femoral artery to carry out the angiographies necessary to monitor the procedure. A transesophageal echocardiogram (TEE) was done during implantation for better anatomical characterization and, in cases of dissection, to clarify the relationship between the true and false lumen, as well as the entry ports. The latter procedures were carried out via intravascular TEE with a Sonicath Ultra G® catheter at 12.5 MHz and a Galaxy2® system (Boston Scientific).
After administering anticoagulation treatment with 10 000 U sodium heparin, the stent was introduced through the arteriotomy and advanced under radiological guidance. Once placed in the correct position and before release, controlled hypotension was induced to minimize the possible displacement of the stent due to heart beat. After release, it was sometimes necessary to inflate a balloon catheter (Medtronic AVE, ReliantTM) inside the stent to shape its components. Once the procedure was completed and the arteriotomy closed, the patients were transferred to the cardiology care unit (CCU) from the cardiology service or to the resuscitation unit depending on the patient's characteristics. Implantation was considered successful when the stent was advanced and delivered in the desired position.
Postimplantation Follow-up Protocol
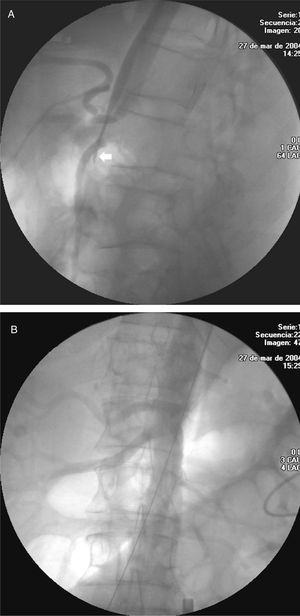
Computed tomographic angiography was done a week after implantation. The parameters under evaluation were: the presence of leaks, absence of flow in the aneurysmatic sac, stent position, and diameter and morphology of the proximal and distal aorta. If there was no leak, a new follow-up was carried out at 6, 12, and 18 months, and annually thereafter. In the patients treated for dissection, perfusion of the abdominal vessels was specifically evaluated and, in particular, those dependent on the false lumen (Figures 3 A and B).
Figure 3. A: celiac trunk severely affected in a patient with acute aortic dissection. Note the collapse of the true lumen which even disappears (arrow). B: permeability of all the branches after the procedure.
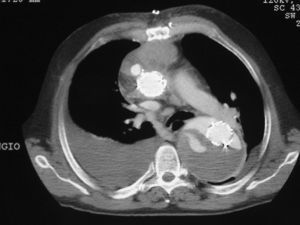
Leaks were classified into the following types: type I, when they were produced in the aortic wall at the proximal or distal insertion points of the stents; type II, when flow appeared in the part of the aorta excluded by the stent through an arterial branch; type III, when the leak occurred through the junction of the stents in the event that more than one was implanted; and type IV, due to stent porosity (Figure 4). In addition, they were classified as early when they were detected between the moment of implantation and the first 6 months of follow-up, and as late when they were detected from 6 months onward following implantation.
Figure 4. Type III endoleaks in several locations. Angiographic CT image where with anterior mediastinal hematoma and a stent located in the ascending aortal arch and the descending aorta, placed within the aortic aneurysm. The aneurysm is not excluded with contrast filling of the false and true lumen.
When a leak was detected during the first examination another was done after 1 month. If the leak persisted and there were suspicions that it was different from a reentry, angiography was carried out and the approach followed was decided on an individual basis. When the leak was considered to be due to reentry, the evolution of the aneurysm was evaluated before proceeding with new invasive procedures.
RESULTS
Immediate or In-Hospital
In 1 of the 15 patients selected, the stent could not be implanted as it was impossible to advance it through an 8-mm diameter Dacron aorto-bifemoral bypass implanted years before. The patient had a ruptured atherosclerotic aneurysm and was transferred from the hemodynamics laboratory to the cardiac surgery operating theater for aorta replacement. Sixteen procedures were done in the remaining 14 patients. The mean age of the patients was 64±9.5 years. The remaining epidemiological characteristics are summarized in Table 1. The mean length of aorta covered was 230±110 mm (range, 110-440 mm). The mean number of stents implanted per patient was 2.3 (1 in 4 patients; 2 in 6 patients; 3 in 1 patient; 4 in 2 patients, and 5 in 1 patient).
One patient had a stent in the ascending aorta and chronic dissection of the descending aorta. During implantation of the stent in the aortic arch the true lumen ruptured in the descending aorta, with a massive passage of blood into the false lumen necessitating the implantation of 2 more stents.
Due to the anatomical characteristics, in 6 cases the stent was implanted immediately distal to the origin of the left carotid artery, occluding the origin of the subclavian. The mean stay in the CCU/resuscitation unit was 2.4 days (range, 2-6), with a mean hospital stay of 11 days (range, 7-25). Control CT carried out a week after the implantation revealed 2 type I endoleaks, 1 type II, and 1 thrombosis of the superior mesenteric artery.
Complications
One death occurred during hospital stay. This involved an 80-year-old patient with chronic kidney failure who, a week after the implantation, developed symptoms of dyspnea and rapidly evolving respiratory failure that did not respond to treatment. A necroscopy to confirm the diagnosis of suspicion of pulmonary thromboembolism could not be done. There were no instances of stroke, arterial thromboembolism or any type of neurological disorder in the remaining patients. One patient presented an elevated ST segment in the electrocardiogram during implantation. At this time, coronary angiography showed an occlusion of the right coronary artery, which was repaired via angioplasty by implanting a sirolimus-eluting stent. Three (20%) patients presented postimplantation syndrome with fever and leukocytosis without a clearly defined focus. All resolved spontaneously. Access route complications included one hematoma of the soft parts that did not require drainage. Two (13.3%) patients developed anemia due to gastrointestinal hemorrhage, one of whom had deterioration of the renal function. A transfusion was necessary in both cases. After implantation, no patient had to be sent back to surgery during their hospital stay.
Follow-up
The mean follow-up was 8.4 months (7 days to 29 months). One patient came back after 8 months due to hemoptysis and chest pain. CT revealed an intramural hematoma that was treated by implanting a new stent, with favorable evolution. In another patient, the reason for consultation was intermittent claudication and impotence 3 months after implantation.
Two cases of early endoleaks were detected more than 1 week after implantation, one of them at 31 days, with the patient presenting chest pain and anemia. The CT study showed type III endoleaks in several locations. After studying the case, it was decided that a new intervention was not suitable and a conservative approach was adopted; the patient was still stable at the time of the last follow-up.
Computerized tomography carried out at 3 months after implantation revealed a small type II endoleak in the distal part of the stent due to filling of the intercostal arteries. No case of stent migration was detected nor were there any deaths during follow-up. The evolution data, together with etiology and follow-up, are summarized in Table 3.
DISCUSSION
Mortality due to elective surgery of thoracic aortic aneurysms fluctuates, depending on the series, between 5% and 20%.4,5,7,8 Emergent interventions in rupture situations have an even higher mortality.9,10
The age of the affected population and the frequent associated comorbidity, as well as morbidity, indicates high surgical risk, especially regarding neurological complications. Stroke and paraplegia or paraparesis continue to be the most feared complications in this type of surgery.11-13 The factors associated with the risk of medullary lesion are the length of the aneurysm, how long the aorta was clamped and the presence of diabetes.14,15
In the last decade, the therapeutic management of this disorder has undergone marked progress. Treatment with stents is raising more interest due to being a less invasive procedure which can be carried out with regional anesthesia or even local anesthesia in the case of the abdominal aorta.16,17
Although the available data are limited, intraluminal repair may become, as some authors point out, the treatment of choice in the majority of patients with lesions in the thoracic aorta. There is growing evidence that this new technique, in the hands of a coordinated team, is a valid and effective alternative to surgery or medical treatment. Thus, several published series indicate perioperative mortality between 0% and 18%, the latter using Endofit devices, the results therefore being discouraging.3,18 During follow-up, mortality ranges from 2% to 17%.19-21 In our series the results were favorable after a mean follow-up of 8.4 months with a hospital mortality of 6% and no more deaths during follow-up.
In the literature, and depending on the series, the incidence of paraparesis exceeds 5%. Its development seems to be related to embolization, blood loss and insufficient collateral circulation.2,3,22,23 The incidence increases if thoracic and abdominal repair are carried out at the same time.24 In our experience, only one case of intermittent claudication and impotence occurred several months after the procedure. It is noteworthy that in two of the selected cases, before stent implantation, bypass surgery was carried out from the ascending aorta to the brachycephalic trunk and left carotid artery to allow the delivery of the stent, which opens up the possibility of endovascular stent-grafting when the aortic arch is included.
One aspect that should be taken into account, which other authors have already emphasized, is the vital importance of the suitable selection of patients and precise knowledge of anatomy.2 Unsuitable vascular access or excessive tortuosity of the aortic arch should be recognized and avoided.
The appearance of endoleaks during follow-up, as occurred in 2 of our patients, seems to be related to alterations in stent placement due to changes in the diseased aortic wall, such as dilatation. On the other hand, as in the series of Fattori et al,2 we did not encounter complications in the patients with postraumatic aneurysm, in whom the device was placed on a normal aortic wall. In cases of type B dissections, for the time being, it seems sensible to reserve the use of stents for patients with significant dilatation, imminent rupture, signs of ischemia or in whom medical treatment has failed. Some authors have already pointed out the need for a random study regarding medical treatment to find out what would be the most appropriate approach for these patients.25
CONCLUSIONS
The results of both the published series and ours suggest that endovascular stent-grafting is a potential way of treating thoracic aorta disorders as it is both effective and safe and can be implemented in high-risk surgical patients.1-4,19-28 Its use requires, as pointed out earlier, the coordinated effort of several medical specialties and appropriate patient selection. However, as all studies suggest, close and long-term follow-up is needed to be certain about the reliability and durability of this new procedure.
Correspondence: Dr. C. Morís.
Unidad de Hemodinámica y Cardiología Intervencionista. Servicio de Cardiología. Hospital Universitario Central de Asturias.
Julian Clavería, s/n. 33006 Oviedo. España.
E-mail: cesar.moris@sespa.princast.es