There is little information on the use of transcatheter aortic valve implantation in patients with severe aortic stenosis and porcelain aorta. The primary aim of this study was to analyze death from any cause after CoreValve® implantation in patients with severe aortic stenosis, with and without porcelain aorta.
MethodsIn this multicenter, observational prospective study, carried out in 3 hospitals, percutaneous aortic valves were implanted in 449 patients with severely calcified aortic stenosis. Of these, 36 (8%) met the criteria for porcelain aorta. The primary end-point was death from any cause at 2 years.
ResultsPatients with porcelain aorta more frequently had extracardiac vascular disease (11 [30.6%] vs 49 [11.9%]; P=.002), prior coronary revascularization (15 [41.7%] vs 98 [23.7%]; P=.017), and dyslipidemia (26 [72.2%] vs 186 [45%]; P=.02). In these patients, there was greater use of general anesthesia (15 [41.7%] vs 111 [16.9%]; P=.058) and axillary access (9 [25%] vs 34 [8.2%]; P=.004). The success rate of the procedure (94.4 vs 97.3%; P=.28) and the incidence of complications (7 [19.4%] vs 48 [11.6%]; P=.20) were similar in both groups. There were no statistically significant differences in the primary end point at 24 months of follow-up (8 [22.2%] vs 66 [16%]; P=.33). The only predictive variable for the primary end point was the presence of complications during implantation (hazard ratio=2.6; 95% confidence interval, 1.5-4.5; P=.001).
ConclusionsIn patients with aortic stenosis and porcelain aorta unsuitable for surgery, percutaneous implantation of the CoreValve® self-expanding valve prosthesis is safe and feasible.
Keywords
Aortic stenosis is the most frequent valvular heart disease in the West. The main cause is degenerative calcification of the aortic valve, and its incidence is constantly increasing due to greater life expectancy.1–3 In the last few years, transcatheter aortic valve implantation (TAVI) has become established as a safe and effective alternative to the surgical treatment of severe symptomatic aortic stenosis in patients unable to undergo surgery or at high surgical risk.4–8 Because this technique has been extensively developed and is constantly innovated, the time may soon be ripe to expand its indications.9
Validated scales are used in these patients to assess surgical risk; the most widely employed are the EuroSCORE (European system for cardiac operative risk evaluation) and the STS (Society of Thoracic Surgeons) risk of mortality score,2–9 but these scales do not include certain clinical characteristics, such as porcelain aorta (AoP).2,10
Patients with porcelain are frequently unsuitable for surgery due to its high technical complexity and the elevated rate of complications, particularly cerebrovascular embolisms.11
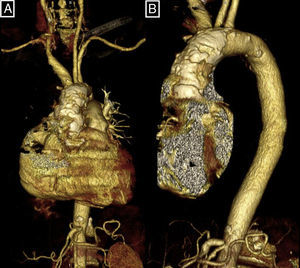
AoP is a structural disease of the aortic wall defined as extensive and circumferential calcium deposition in the thoracic aorta (Fig. 1), detected by computed tomography or fluoroscopy.11,12
The frequency of this entity in patients with aortic stenosis treated with TAVI varies from 10% to 35%2,12; AoP and poor vascular access are the two main reasons for indicating transapical access in TAVI.13
Two surgical alternatives to the classical treatment of aortic stenosis with concomitant AoP are the apicoaortic conduit and “sutureless” aortic valve replacement, but these techniques lack demonstrated efficacy.14,15 AoP is sometimes unnoticed, and are only detected as a surgical finding after sternotomy.16–18
There is scarce information on the results of TAVI in patients with AoP, and published data are limited to case reports or small series.11,13,16,19 Although most published series have included patients with AoP, this entity has not been studied specifically.2,11,13
The aim of this study was to determine death from any cause after TAVI in patients with severe symptomatic aortic stenosis and AoP and to compare the results with those obtained in patients without a severely calcified aorta.
METHODSThis multicenter, observational, prospective study included data from patients with severe symptomatic aortic stenosis treated with TAVI from December, 2007 to April 2012 in 3 Spanish hospitals: Hospital Universitario Virgen de la Victoria de Málaga (233 patients, 19 with AoP), Hospital Clínico Universitario de Santiago de Compostela (101 patients, 9 with AoP), and Hospital Universitario Central de Asturias (115 patients, 8 with AoP).
Data on clinical features, the procedure, and follow-up were gathered using a preestablished protocol common to the 3 hospitals and were later entered into a central database specifically designed for this study. The follow-up consisted of a cardiologist visit.
All patients had severe symptomatic aortic stenosis. Additional inclusion criteria were as follows: aortic valve area <1 cm2 (<0.6 cm2/m2); aortic annulus between 20 and 27mm; diameter of the ascending aorta at the sinotubular junction≤40mm (26-mm prosthesis) or ≤43 mm (29-mm prosthesis).
All patients were evaluated by a multidisciplinary team composed of clinical cardiologists, interventional cardiologists, and cardiac surgeons who determined the indication for TAVI. This team was also responsible for confirming the presence of AoP and determining the contraindication to surgery due to AoP. At least two cardiac surgeons forming part of the team had to agree that the patient was not a suitable candidate for surgery. Diagnosis of AoP was performed by computed tomography (the presence of circumferential calcification of the ascending aorta) or fluoroscopy (the presence of diffuse, general calcification of the walls of the ascending aorta in at least 2 orthogonal projections).2,4,5,10,12,13,17 Two patients were referred to TAVI after sternotomy due to the presence of AoP that prevented aortic clamping.
Before the prosthesis was implanted, all patients underwent the following procedures: coronary angiography, aortic angiography of the ascending aorta, angiography of the iliofemoral region, and transthoracic and transesophageal echocardiography. Contrast-enhanced computed tomography was performed when deemed necessary by the cardiology team. The logistic EuroSCORE risk scale was used to evaluate surgical risk.
Transcatheter Aortic Valve ImplantationThe prosthesis used was the CoreValve ReValving System® by Medtronic (26-mm and 29-mm sizes). Implantation was carried out in the cardiac catheterization laboratory under general anesthesia or local anesthesia and deep sedation, as deemed suitable by the anesthesiologist. All patients received prophylactic antibiotic coverage with cephalosporins, except those allergic to beta-lactams, who received vancomycin. Vascular access was through the femoral route, except when prevented by the iliofemoral axis, in which case access was gained through the axillary route. The details of the implantation technique and the hospital management of the patients have been previously described.7,8 The patients were followed-up at 30 days and again at 6 months. To analyze the results, we used the definitions of the VARC (Valve Academic Research Consortium) consensus report.20
Primary End PointDeath from any cause in patients with severe aortic stenosis with and without AoP undergoing TAVI with a CoreValve® prosthesis at 2 years.
DefinitionsProcedural success: correct implantation and functioning of the prosthesis (evaluated by angiography and echocardiography) in patients surviving the procedure.
Vascular complications: aortic dissection, aortic annulus rupture, failure of the percutaneous closure device, or iliac or femoral rupture.
Life-threatening bleeding: hemorrhages in the critical organ or provoking hypovolemic shock or a drop in hemoglobin of greater than 5g or requiring transfusion of more than 4 blood units.
Death from any cause: death from any cause occurring during the follow-up.
Cardiovascular mortality: mortality meeting at least 1 of the following criteria: any death due to a cardiac cause, unexpected death or death from an unknown cause, death related to a complication or the treatment of a complication resulting from TAVI, and death from a noncoronary vascular cause.
Thirty-day mortality: death from any cause occurring within 30 days of TAVI and directly due to the complications of the procedure or to the treatment of a TAVI-related complication.
Statistical analysisThe Kolmogorov-Smirnov test was used to verify the normality of the distribution of the variables. The data are expressed as the mean (standard deviation) for continuos variables and as numbers and percentages for categorical variables. The data are presented by comparing two groups: patients with or without AoP. Continuous quantitative variables were compared using Student's t test or the Mann-Whitney U-test and proportions were compared with the chi-square test. A multivariable analysis was carried out to analyze the factors independently predictive of the primary end point (death from any cause at 2 years) through a Cox survival analysis that included age, sex, the presence of AoP, and the variables associated with mortality (P<.2) in the univariable analysis. A survival study was performed using Kaplan-Meier analysis. The log rank test was used to compare survival between the groups with and without AoP. The statistical analysis was performed with the SPSS statistical program, version 20 (SPSS Inc.; Chicago, Illinois, United States).
RESULTSCharacteristics of the PopulationBetween December, 2007 and April, 2012, 449 patients with severe calcified aortic stenosis were consecutively treated with CoreValve® implantation (233 in Hospital Clínico Virgen de la Victoria de Málaga, 115 in Hospital Universitario Central de Asturias and 101 in Hospital Clínico Universitario de Santiago de Compostela). Of these, 36 (8%) met the criteria for a diagnosis of AoP (19, 8, and 9 from each hospital, respectively). All diagnoses were based on the results of fluoroscopy. Computed tomography was performed in 6% of patients without AoP and in 25% of those with AoP.
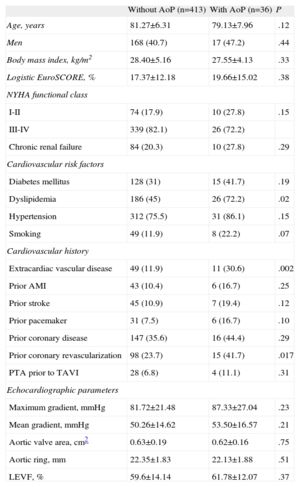
The baseline characteristics of the population are shown in in Table 1. The presence of extracardiac vascular disease was more frequent in the group with AoP (11 [30.6%] vs 49 [11.9%]; P=.002), as was prior coronary revascularization (15 [41.7%] vs 98 [23.7%]; P=.017), and a history of dyslipidemia (26 [72.2%] vs 186 [45%]; P=.02). There were no significant differences between the two groups in the remaining clinical and echocardiographic variables.
Baseline Characteristics of the Sample (n=449)
| Without AoP (n=413) | With AoP (n=36) | P | |
| Age, years | 81.27±6.31 | 79.13±7.96 | .12 |
| Men | 168 (40.7) | 17 (47.2) | .44 |
| Body mass index, kg/m2 | 28.40±5.16 | 27.55±4.13 | .33 |
| Logistic EuroSCORE, % | 17.37±12.18 | 19.66±15.02 | .38 |
| NYHA functional class | |||
| I-II | 74 (17.9) | 10 (27.8) | .15 |
| III-IV | 339 (82.1) | 26 (72.2) | |
| Chronic renal failure | 84 (20.3) | 10 (27.8) | .29 |
| Cardiovascular risk factors | |||
| Diabetes mellitus | 128 (31) | 15 (41.7) | .19 |
| Dyslipidemia | 186 (45) | 26 (72.2) | .02 |
| Hypertension | 312 (75.5) | 31 (86.1) | .15 |
| Smoking | 49 (11.9) | 8 (22.2) | .07 |
| Cardiovascular history | |||
| Extracardiac vascular disease | 49 (11.9) | 11 (30.6) | .002 |
| Prior AMI | 43 (10.4) | 6 (16.7) | .25 |
| Prior stroke | 45 (10.9) | 7 (19.4) | .12 |
| Prior pacemaker | 31 (7.5) | 6 (16.7) | .10 |
| Prior coronary disease | 147 (35.6) | 16 (44.4) | .29 |
| Prior coronary revascularization | 98 (23.7) | 15 (41.7) | .017 |
| PTA prior to TAVI | 28 (6.8) | 4 (11.1) | .31 |
| Echocardiographic parameters | |||
| Maximum gradient, mmHg | 81.72±21.48 | 87.33±27.04 | .23 |
| Mean gradient, mmHg | 50.26±14.62 | 53.50±16.57 | .21 |
| Aortic valve area, cm2 | 0.63±0.19 | 0.62±0.16 | .75 |
| Aortic ring, mm | 22.35±1.83 | 22.13±1.88 | .51 |
| LEVF, % | 59.6±14.14 | 61.78±12.07 | .37 |
AMI, acute myocardial infarction; EuroSCORE, European system for cardiac operative risk evaluation score; LEVF, left ventricular ejection fraction; NYHA, New York Heart Association; PTA, percutaneous transluminal angioplasty; TAVI, transcatheter aortic valve implantation.
The data are expressed as no. (%) or mean±standard deviation.
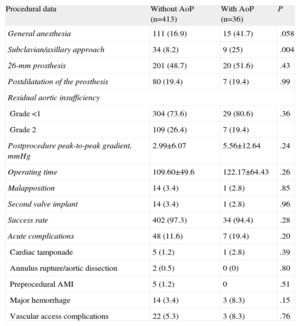
Data on the procedure are show in Table 2. General anesthesia was more frequently used in patients with AoP (—15 [41.7%] vs 111 [16.9%]; P=.058), as was the axillary approach (9 [25%] vs 34 [8.2%]; P=.004). In both groups, the most frequent size of the prosthesis was 26mm. The mean operating time showed no significant differences but was higher in the group with AoP. The success rate was similar in both groups (97.3% vs 94%). There were no differences in the percentage of malapposition, or in any of the complications due to the procedure (7 [19.4%] vs 48 [11.6%]; P=.20). None of the patients required conversion to conventional surgery. There were no significant differences in the remaining complications.
Data on Transcatheter Aortic Valve Implantation and Acute Complications
| Procedural data | Without AoP (n=413) | With AoP (n=36) | P |
| General anesthesia | 111 (16.9) | 15 (41.7) | .058 |
| Subclavian/axillary approach | 34 (8.2) | 9 (25) | .004 |
| 26-mm prosthesis | 201 (48.7) | 20 (51.6) | .43 |
| Postdilatation of the prosthesis | 80 (19.4) | 7 (19.4) | .99 |
| Residual aortic insufficiency | |||
| Grade <1 | 304 (73.6) | 29 (80.6) | .36 |
| Grade 2 | 109 (26.4) | 7 (19.4) | |
| Postprocedure peak-to-peak gradient, mmHg | 2.99±6.07 | 5.56±12.64 | .24 |
| Operating time | 109.60±49.6 | 122.17±64.43 | .26 |
| Malapposition | 14 (3.4) | 1 (2.8) | .85 |
| Second valve implant | 14 (3.4) | 1 (2.8) | .96 |
| Success rate | 402 (97.3) | 34 (94.4) | .28 |
| Acute complications | 48 (11.6) | 7 (19.4) | .20 |
| Cardiac tamponade | 5 (1.2) | 1 (2.8) | .39 |
| Annulus rupture/aortic dissection | 2 (0.5) | 0 (0) | .80 |
| Preprocedural AMI | 5 (1.2) | 0 | .51 |
| Major hemorrhage | 14 (3.4) | 3 (8.3) | .15 |
| Vascular access complications | 22 (5.3) | 3 (8.3) | .76 |
AMI, acute myocardial infarction; AoP, porcelain aorta.
Data are expressed as no. (%) or mean±standard deviation.
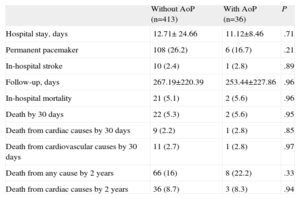
There were no differences between the groups in in-hospital mortality (21 [5.1%] vs 2 [5.6%]; P=.96) or in 30-day mortality (22 [5.3%] vs 2 [5.6%]; P=.95).
In-hospital mortality (n=21) in the group without AoP was due to the following causes: heart failure (n=3), multiorgan failure (n=5), cardiorespiratory arrest with asystole (n=3), severe acute renal insufficiency (n=1), pulmonary hemorrhage (n=1), mesenteric ischemia (n=2), perforation (n=1), cardiac rupture (n=1), fatal vascular hemorrhage (n=1), stroke (n=1), and sepsis (n=2). In the group with AoP, hospital mortality (n=2) was due to massive hemothorax (n=1) and heart failure (n=1).
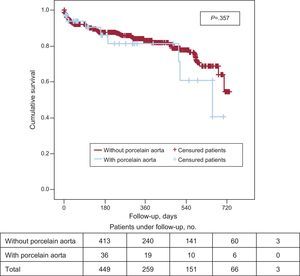
Primary End PointFollow-up data were available in all patients. The mean length of follow-up was 241 days (interquartile range, 34.5-434). There were no differences between the two groups in the follow-up period (Table 3). Death from any cause was 74 patients (16.5%), with no differences between the groups with and without AoP (8 [22%] vs 66 [16%]; P=.33).
Follow-up Data
| Without AoP (n=413) | With AoP (n=36) | P | |
| Hospital stay, days | 12.71± 24.66 | 11.12±8.46 | .71 |
| Permanent pacemaker | 108 (26.2) | 6 (16.7) | .21 |
| In-hospital stroke | 10 (2.4) | 1 (2.8) | .89 |
| Follow-up, days | 267.19±220.39 | 253.44±227.86 | .96 |
| In-hospital mortality | 21 (5.1) | 2 (5.6) | .96 |
| Death by 30 days | 22 (5.3) | 2 (5.6) | .95 |
| Death from cardiac causes by 30 days | 9 (2.2) | 1 (2.8) | .85 |
| Death from cardiovascular causes by 30 days | 11 (2.7) | 1 (2.8) | .97 |
| Death from any cause by 2 years | 66 (16) | 8 (22.2) | .33 |
| Death from cardiac causes by 2 years | 36 (8.7) | 3 (8.3) | .94 |
AoP, porcelain aorta.
Data are expressed as no. (%) or mean±standard deviation.
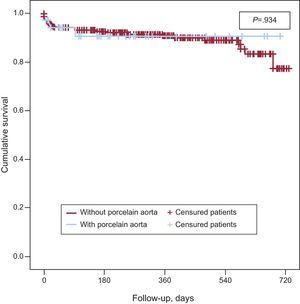
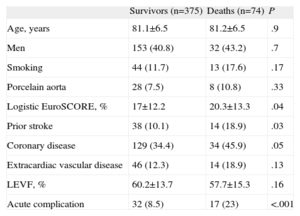
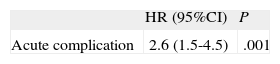
Kaplan-Meier survival analysis revealed no significant differences in 2-year survival (Fig. 2) between the 2 groups (P=.35) with a survival of 40.7% (mean survival, 544 [47 days]; 95% confidence interval [95%CI], 452-638 days) in the group with AoP and of 54.8% in the group without AoP (mean survival, 593 [14] days; 95%CI, 565-622 days). There were no differences between the 2 groups in mortality from cardiac causes (Fig. 3). The results of the univariable and multivariable analyses with the predictors of the primary end point are shown in Tables 4 and 5. The only predictor of mortality was the presence of at least 1 acute complication during implantation (odds ratio=2.6; 95%CI, 1.5-4.5; P=.001).
Univariable Analysis Showing the a Statistically Significant Association or Trend With Mortality, Together With Age, Sex and the Presence of Porcelain Aorta (n=449)
| Survivors (n=375) | Deaths (n=74) | P | |
| Age, years | 81.1±6.5 | 81.2±6.5 | .9 |
| Men | 153 (40.8) | 32 (43.2) | .7 |
| Smoking | 44 (11.7) | 13 (17.6) | .17 |
| Porcelain aorta | 28 (7.5) | 8 (10.8) | .33 |
| Logistic EuroSCORE, % | 17±12.2 | 20.3±13.3 | .04 |
| Prior stroke | 38 (10.1) | 14 (18.9) | .03 |
| Coronary disease | 129 (34.4) | 34 (45.9) | .05 |
| Extracardiac vascular disease | 46 (12.3) | 14 (18.9) | .13 |
| LEVF, % | 60.2±13.7 | 57.7±15.3 | .16 |
| Acute complication | 32 (8.5) | 17 (23) | <.001 |
EuroSCORE, European system for cardiac operative risk evaluation score; LEVF, left ventricular ejection fraction.
Data are expressed as no. (%) or mean±standard deviation.
Multivariable Analysis With Predictors of Mortality
| HR (95%CI) | P | |
| Acute complication | 2.6 (1.5-4.5) | .001 |
95%CI, 95% confidence interval; HR, hazard ratio.
Variables not predictive of the primary end point were age, sex, porcelain aorta, logistic EuroSCORE, the presence of coronary disease, smoking, prior stroke, extracardiac vascular disease and left ventricular ejection fraction.
The main finding of this study is that, in patients with severe aortic stenosis who cannot undergo surgery due to AoP, percutaneous CoreValve® implantation is safe and feasible and has similar success and complications rates to those obtained in patients without aortic involvement. Ours is the largest series of patients with severe aortic stenosis and AoP treated with TAVI reported to date.
The combination of AoP and aortic stenosis makes valve replacement surgery technically difficult and strongly influences morbidity and mortality.21–27
All patients in this series were excluded from surgery by a multidisciplinary group due to their high surgical risk and the presence of AoP. The scale most widely used in Spain to evaluate surgical risk in such patients is the EuroSCORE, which does not include AoP and consequently fails to reflect their real surgical risk.
As reflected by our findings, the most frequent indication for TAVI in our environment continues to be a high EuroSCORE. In our series, the EuroSCOREs were similar in both groups and were also similar to those in other recently published multicenter studies,7,8,28 but were lower than those in earlier reports.2,4–6
The prevalence of AoP in TAVI varies widely, oscillating between 10% and 35%. This variability could be explained by the wide heterogeneity in the criteria used to diagnose this entity.2,5,11–13 In our series, the prevalence of AoP was lower than that reported in other studies,2,11,29 possibly because our study was carried out in 3 hospitals with a uniform definition of AoP, a substantial volume of activity, and extensive experience of TAVI.
In this series, the higher use of the axillary approach in patients with AoP could have been related to the higher presence of extracardiac vascular disease (30.6% vs 11.9%), which could also explain the lower percentage of vascular complications in the group with AoP. In the last few years, several studies have reported the axillary route to be safe and effective in TAVI in patients with poor femoral access.29,30
The success rate was similar in both groups. These results confirm those of previous series of TAVI that included patients with AoP.2,7,8,28,31,32 Prior publications had suggested the possibility that there might be a greater tendency to malapposition in patients with AoP undergoing TAVI,2,11,12 but this tendency was not observed in our series.
The most common complication in this study was the need for pacemaker implantation but the frequency of this complication was similar in the groups with or without AoP. This finding is in agreement with the results of other studies performed in patients with the CoreValve® prosthesis.7,8 As noted by other studies,33–35 this complication seems mainly related to low CoreValve® implantation and the presence of prior conduction alterations or right bundle branch block.33–35
In this study, we included only strokes with clinical repercussions. The incidence of stroke was very low and was similar in patients with and without AoP. However, the frequency of clinically silent cerebral embolic lesions might have been higher if we had systematically carried out independent neurological evaluation and had used high-resolution imaging techniques immediately after TAVI.36–38
Mortality at 1 month was also low, with no differences between the 2 groups. As in prior series of TAVI,7,8,28,32 the main cause of death within 30 days was procedure-related complications. Nevertheless, mortality was lower than the expected mortality, as estimated by the logistic EuroSCORE algorithm.38,39 There was no difference in long-term between the 2 groups. The survival function in this study could be superimposed over that of other works with a similar design but which used transapical access in patients with severe calcification of the ascending aorta.11
In general, the results of this study show that treatment with TAVI may be a worthwhile therapeutic alternative to conventional surgery or to surgical options through axillary artery cannulation, apicoaortic conduit, or ascending aortic replacement.14,40–42
LimitationsThe number of patients included in this study was small and the follow-up was short. Consequently, our study may lack the statistical power needed to detect differences in the incidence of some complications. Although the VARC criteria were used to analyze cerebrovascular complications, neurological evaluation was not systematically carried out, which could have led to underestimation of these complications.
The data presented refer to the results of 3 centers with high volume and extensive experience of CoreValve® implantation and may not therefore be suitable for extrapolation to centers with a lower patient volume or those using other devices.
CONCLUSIONSPercutaneous CoreValve® implantation in patients with AoP who are not deemed suitable candidates for surgery is safe and feasible. The results obtained in this study did not differ from those obtained in patients without diffuse calcification of the ascending aorta.
Our results indicate that, in patients with severe aortic stenosis and AoP, classically associated with high surgical risk, TAVI is an attractive therapeutic option.